Reticular Materials Gain Traction in Carbon Capture Technologies: Reticular materials have become a pivotal innovation in carbon capture technologies, offering promising solutions to mitigate climate change. These advanced materials—especially Metal-Organic Frameworks (MOFs) and Covalent Organic Frameworks (COFs)—are capturing attention for their unique ability to efficiently trap carbon dioxide (CO₂). This article explores the science behind reticular materials, their advantages in carbon capture, and how they might reshape the future of environmental technology.
Whether you are a student, a curious reader, or a professional in the energy or environmental sector, this guide will help you understand the role of reticular materials in carbon capture—breaking down complex concepts into clear, actionable insights.
Table of Contents
Reticular Materials Gain Traction in Carbon Capture Technologies
| Feature | Details |
|---|---|
| Surface Area | Up to 7,000 m²/g in some MOFs |
| CO₂ Capture Capacity | 1–4 mmol/g under typical conditions |
| Regeneration Temperature | As low as 60°C for some COFs (e.g., COF-999) |
| Durability | Stable in steam and acidic gases for extended periods |
| Industrial Adoption | Startups like Atoco developing DAC modules with reticular materials |
Reticular materials are reshaping the future of carbon capture with their high selectivity, large surface area, low energy regeneration, and durability. Their integration into industrial and environmental applications promises a cost-effective and scalable path to reducing CO₂ emissions globally.
By harnessing advanced chemistry and AI-driven innovation, reticular materials are poised to become vital tools in the fight against climate change, supporting cleaner industries and helping meet ambitious climate goals.
For detailed technology information, visit Atoco’s official site.
Historical Context: How Carbon Capture Evolved
Carbon capture technology has been evolving for decades, starting with chemical solvents like amines to absorb CO₂ from industrial emissions. While effective, these traditional methods often come with high energy consumption and operational costs.
In recent years, scientists have turned to materials science to develop alternatives that can capture CO₂ more efficiently. This quest led to the discovery and development of reticular materials, which stand out due to their highly porous structure and chemical tunability.
What Are Reticular Materials?
Reticular materials are crystalline, porous frameworks constructed by linking molecular building blocks through strong chemical bonds.
Metal-Organic Frameworks (MOFs)
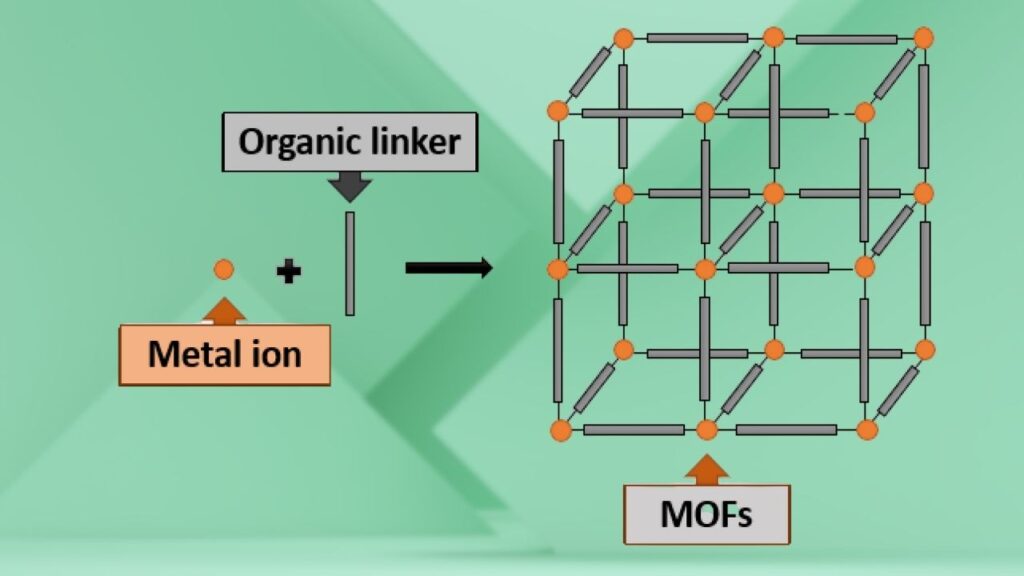
MOFs are composed of metal ions or clusters connected by organic linkers, creating a vast internal surface area—sometimes exceeding 7,000 square meters per gram, enough to cover several football fields. This enormous surface area allows MOFs to trap significant amounts of CO₂.
Covalent Organic Frameworks (COFs)
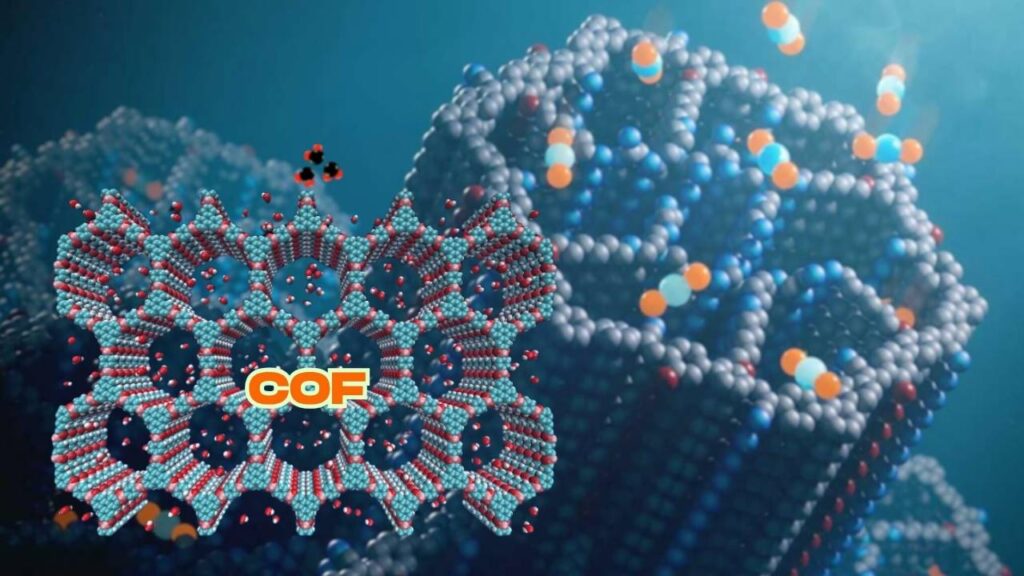
COFs consist entirely of organic molecules joined by covalent bonds. They offer excellent thermal and chemical stability, making them suitable for tough industrial environments.
Why Reticular Materials Matter in Carbon Capture
The unique properties of reticular materials make them ideal for capturing carbon dioxide:
- Selective Adsorption: Their pores can be designed to preferentially attract CO₂ molecules while excluding other gases like nitrogen.
- Energy-Efficient Regeneration: Unlike traditional solvents that require high heat, some COFs can release CO₂ at temperatures as low as 60°C, significantly reducing energy usage.
- Durability: Certain MOFs and COFs remain stable under moisture and acidic conditions, enabling long-term industrial use.
- Scalability: Advances in synthesis are making it possible to produce these materials at scale and lower costs.
How Reticular Materials Capture CO₂: A Step-By-Step Guide
Step 1: Adsorption
Flue gas or air containing CO₂ passes through a reticular material bed. CO₂ molecules are trapped inside the material’s microscopic pores, while other gases pass through.
Step 2: Saturation
The material fills up with CO₂ molecules, depending on its surface area and pore size.
Step 3: Regeneration
By applying gentle heat (e.g., around 60°C for some COFs), the CO₂ is released from the material in a concentrated form, ready for storage or utilization.
Step 4: Reuse
The reticular material is reusable over many cycles with minimal loss of efficiency.
Practical Applications and Industry Impact
Reticular materials are revolutionizing several carbon capture applications:
- Post-Combustion Capture: Efficiently scrubbing CO₂ from power plant emissions.
- Direct Air Capture (DAC): Extracting CO₂ directly from the ambient air, enabling negative emissions.
- Natural Gas Processing: Purifying natural gas by removing CO₂.
- Chemical Industry: Capturing CO₂ for reuse in producing chemicals and fuels.
Example: Atoco, co-founded by MOF pioneer Professor Omar Yaghi, develops solid-state carbon capture modules using reticular materials designed for both DAC and industrial exhaust. Their technology promises lower costs and energy use compared to traditional systems.
Environmental Impact: A Step Toward Climate Goals
Adoption of reticular materials in carbon capture can significantly reduce global CO₂ emissions. The International Energy Agency (IEA) estimates that carbon capture technologies could remove up to 15% of global CO₂ emissions by 2050.

By making carbon capture more efficient and affordable, reticular materials support cleaner energy transitions and contribute to international climate commitments under the Paris Agreement.
Economic Considerations
While the initial cost of reticular materials can be higher than conventional absorbents, their energy savings and durability offer lower operational costs in the long run.
- Cost Reduction: Scale-up and manufacturing innovations are expected to reduce MOF and COF production costs by 30-50% in the next decade.
- Return on Investment: Industrial users can expect faster ROI due to lower energy demands and longer material lifespans.
Challenges and Future Directions
Despite their promise, reticular materials face challenges:
- Manufacturing Scale: Large-scale, cost-effective production needs continued development.
- Material Longevity: Long-term stability under real industrial conditions requires ongoing research.
- System Integration: Engineering these materials into existing carbon capture infrastructure remains complex.
Researchers are actively applying artificial intelligence (AI) and machine learning to discover new frameworks with superior CO₂ uptake and stability. Recent studies using GFlowNets have identified materials outperforming existing ones in working capacity.
Regulatory and Policy Influence
Government policies promoting carbon capture and storage (CCS) technologies are crucial for widespread adoption of reticular materials. Incentives such as tax credits (e.g., the U.S. 45Q tax credit) encourage companies to invest in CCS.
Clear regulations and funding for pilot projects will accelerate integration of these advanced materials into commercial operations.
Practical Advice for Industry Adoption
Companies interested in implementing reticular materials for carbon capture should:
- Conduct pilot tests to assess performance under specific emission conditions.
- Partner with material manufacturers to customize MOFs or COFs for their needs.
- Invest in employee training on handling and maintenance of new capture systems.
- Monitor emerging regulatory incentives to optimize project financing.
Real-World Case Studies
- Atoco’s DAC Modules: Demonstrated low-energy CO₂ capture with high material durability.

- Pilot Plants Using MOFs: Several startups have successfully tested MOF-based capture at power plants with promising results.
These examples show growing confidence in reticular materials’ commercial viability.
Glossary of Key Terms
- Adsorption: The process of molecules sticking to a surface.
- Covalent Organic Framework (COF): A porous crystalline material made from organic molecules linked by covalent bonds.
- Metal-Organic Framework (MOF): A porous material made from metal ions connected by organic linkers.
- Direct Air Capture (DAC): Technology to capture CO₂ directly from the atmosphere.
- Regeneration: Releasing captured CO₂ from a material to reuse the material.
European Startups Achieve Breakthroughs in Recycling EV Battery Materials
Robotic Automation Enhances Scalability of 2D Materials for Electronic Devices
Rice University Researchers Discover New 2D Material With Exceptional Adhesion Properties
FAQs About Reticular Materials Gain Traction in Carbon Capture Technologies
Q1: What makes reticular materials better than traditional carbon capture solvents?
A: They selectively capture CO₂ with lower energy needs for regeneration, making the process more efficient and cost-effective.
Q2: Are reticular materials safe for the environment?
A: Yes, they reduce reliance on harsh chemicals and can be reused many times, minimizing waste.
Q3: How soon will reticular materials be widely used?
A: Some applications are already underway, with wider adoption expected in the next 5–10 years as costs decline.
Q4: Can reticular materials capture other greenhouse gases?
A: Primarily designed for CO₂, but research is ongoing to extend their use to other gases like methane.