Prototype Of Sodium-Air Fuel Cell With Promising Efficiency: The development of an early prototype of a sodium-air fuel cell with promising efficiency marks a significant breakthrough in clean energy technology. This advancement, led by researchers at the Massachusetts Institute of Technology (MIT), offers a potential alternative to lithium-ion batteries and traditional fossil fuels for applications demanding high energy density. By combining abundant materials with innovative chemistry, this technology could power electric aircraft, drones, and heavy transportation in an environmentally friendly way.

In this article, we’ll explore what sodium-air fuel cells are, how they work, why their energy density matters, and their potential to reshape the future of transportation and clean energy. This explanation will be approachable for beginners, including young readers, while providing professionals with deep insights and valuable technical details.
Table of Contents
Prototype Of Sodium-Air Fuel Cell With Promising Efficiency
| Aspect | Details |
|---|---|
| Technology | Sodium-air fuel cell prototype |
| Energy Density | Laboratory results show over 1,500 Wh/kg; real-world targets 1,000+ Wh/kg |
| Comparison | Lithium-ion batteries: ~250–300 Wh/kg |
| Developed By | MIT researchers and startup Propel Aero |
| Applications | Drones, electric aviation, trucks, ships, trains |
| Environmental Impact | Zero emissions; byproducts react with CO₂ forming sodium bicarbonate |
| Refueling Method | Replace sodium metal cartridge instead of recharging |
| Operating Temperature | Approximately 90°C (194°F) in prototype |
| Official MIT Source | MIT News Article |
The sodium-air fuel cell prototype developed by MIT researchers represents a significant step toward high-efficiency, environmentally friendly energy storage solutions. With energy densities far exceeding lithium-ion batteries and unique benefits like CO₂ absorption and fast refueling, this technology holds promise for electric aviation, drones, and heavy transport sectors that have long struggled with battery limitations.
Although challenges related to operating temperature, durability, and infrastructure remain, ongoing research and industry interest indicate a strong future for sodium-air fuel cells. As development progresses, this innovation could play a pivotal role in the transition to sustainable, zero-emission transportation and energy systems worldwide.
What Is a Sodium-Air Fuel Cell?

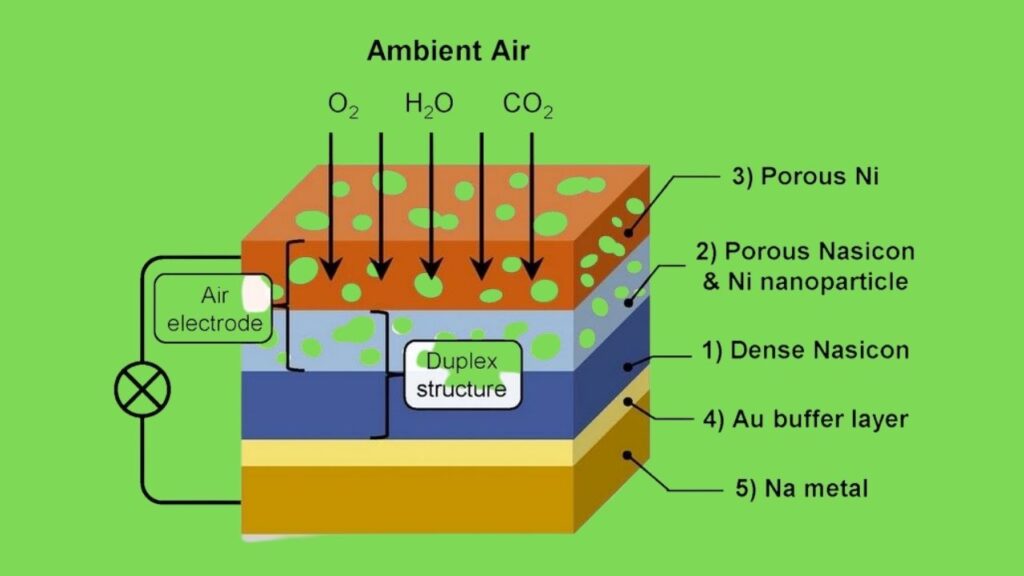
A sodium-air fuel cell is a type of metal-air battery that generates electricity through a chemical reaction between sodium metal and oxygen from the surrounding air. Unlike conventional batteries that store both reactants internally and require recharging, sodium-air cells consume sodium metal as a fuel and draw oxygen continuously from the atmosphere.
The Chemistry Behind It
The fuel cell consists of several key components:
- Liquid sodium metal serves as the anode (fuel source).
- Oxygen from the air serves as the cathode reactant.
- A solid ceramic electrolyte—specifically, a sodium-ion conducting ceramic—separates the two sides and allows sodium ions (Na⁺) to flow through it.
- When operating, sodium at the anode releases electrons and ions.
- The sodium ions migrate through the electrolyte to the cathode, where they react with oxygen and electrons from the external circuit to form sodium oxides.
- These oxides eventually convert into sodium carbonate or bicarbonate, depending on CO₂ presence.
This electrochemical process produces an electric current capable of powering electronic devices or motors.
Why Is This Important?
Most current batteries, like lithium-ion, carry both reactants internally, limiting their energy density and making recharging necessary. Sodium-air cells get oxygen from the air, reducing weight and boosting energy potential. Moreover, sodium is an abundant and inexpensive metal, making the fuel cost-effective.
Understanding Energy Density: Why It Matters
Energy density is the amount of energy stored per unit of weight (Wh/kg). It is critical for transportation applications where weight directly affects performance and efficiency.
How Sodium-Air Compares:
- Lithium-ion batteries: Typical energy density ranges from 250 to 300 Wh/kg.
- Jet fuel: Has an energy density of about 12,000 Wh/kg, but much of that energy is lost in combustion inefficiency.
- Sodium-air fuel cell prototype: Demonstrated >1,500 Wh/kg in laboratory tests, with projected real-world energy density of 1,000 Wh/kg or more.
Reaching 1,000 Wh/kg is a significant benchmark because, according to studies from Boeing and NASA, this level is roughly what electric planes need to operate on mid-range flights effectively.
The Impact of Higher Energy Density
Higher energy density means:
- Longer flight or driving range without increasing battery weight.
- Smaller, lighter power units, freeing up payload capacity.
- Potentially faster refueling, as replacing sodium fuel is quicker than recharging batteries.
This makes sodium-air technology a potential game-changer for electric aviation and other sectors.
Environmental Benefits and Sustainability
One of the most compelling features of this sodium-air fuel cell prototype is its positive environmental impact:
- Zero emissions during operation: The system emits no greenhouse gases or harmful pollutants.
- CO₂ Absorption Potential: The reaction byproducts can chemically bind with carbon dioxide in the environment, producing sodium bicarbonate (baking soda), which is environmentally benign.
- Use of Earth-Abundant Materials: Sodium is the sixth most abundant element in the Earth’s crust, widely available and inexpensive compared to lithium or rare earth metals used in conventional batteries.
- Reduced reliance on mining critical minerals: Lithium and cobalt mining often has high environmental and human costs. Sodium’s abundance can alleviate these issues.
Sodium-Air vs. Lithium-Ion Environmental Impact
While lithium-ion batteries are pivotal to today’s clean energy transition, their environmental footprint includes mining challenges and resource scarcity. Sodium-air fuel cells offer an alternative path that could complement or even replace lithium-ion technology in some applications.
Real-World Applications: From Drones to Electric Planes
1. Drones
Drones require lightweight, high-energy power sources to maximize flight time. Current lithium-ion batteries limit many commercial drones to under an hour of flight.
- Sodium-air fuel cells could enable drones to fly for several hours on a single charge.

- Refueling by swapping sodium cartridges can reduce downtime during operations such as deliveries, surveillance, or agricultural monitoring.
2. Electric Aviation
Electric aircraft are emerging as an essential solution for reducing aviation emissions. However, battery weight and energy density limit their range and payload.
- MIT’s sodium-air prototype is aimed initially at small to medium electric planes, potentially achieving ranges comparable to current fossil fuel planes.
- The fuel cell’s high energy density and fast refueling address two primary barriers to electric flight.
3. Heavy Transport
Trucks, trains, and ships are harder to electrify due to energy demands and long operation times.
- Sodium-air fuel cells may power heavy vehicles more efficiently than batteries by reducing the weight and enabling quick refuel.
- The technology could facilitate cleaner shipping and freight transport, reducing global emissions.
Technical Challenges and Research Frontiers
Despite these promising aspects, several technical hurdles remain:
Operating Temperature
The prototype operates around 90°C (194°F) to maintain sodium in liquid form and optimize ion flow through the ceramic electrolyte.
- Achieving efficient operation at lower temperatures is essential for broader adoption.
- Research continues into advanced electrolytes and thermal management to reduce this barrier.
Durability and Stability
- The ceramic electrolyte and sodium metal must withstand repeated cycles of sodium replacement without degradation.
- Long-term stability under real-world conditions (vibration, temperature variation, contaminants) needs extensive testing.
Refueling Infrastructure
- Developing safe, scalable systems for handling and distributing sodium cartridges is crucial.
- Safety protocols must mitigate sodium’s high reactivity with water and air.
Manufacturing and Cost
- Scaling ceramic electrolyte production while maintaining performance.
- Ensuring overall cost competitiveness compared to existing battery systems.
The Path Forward: Commercialization and Industry Impact
MIT’s startup, Propel Aero, is leading efforts to bring sodium-air fuel cells from the lab to the market. Their roadmap includes:
- Miniaturizing the fuel cell to fit drone sizes within 5 years.
- Collaborating with aerospace partners for certification and regulatory approval.
- Expanding into other sectors like trucks and marine vessels over the next decade.
Investment in this technology is growing, reflecting broader industry trends favoring solid-state batteries, metal-air chemistries, and green hydrogen as future energy carriers.
ISRO Declares 2025 as Gaganyaan Year with December Launch Featuring Humanoid Robot Vyommitra
Berkeley Lab Unveils Doudna Supercomputer to Propel AI and Genomics Research
Universities Partner With Industry To Build The Next Generation Of Sustainable Semiconductors
FAQs About Prototype Of Sodium-Air Fuel Cell With Promising Efficiency
What makes sodium-air fuel cells different from other batteries?
They use sodium metal and oxygen from the air to generate electricity, leading to higher energy density and lighter weight than traditional lithium-ion batteries.
Are sodium-air fuel cells rechargeable?
They are refuelable rather than rechargeable—you replace the sodium metal fuel rather than recharging the battery electrically.
Is sodium safe to use in fuel cells?
While sodium metal is highly reactive, the fuel cell design uses liquid sodium contained safely within a ceramic electrolyte, minimizing hazards.
How soon can this technology be commercially available?
Early commercial applications, especially in drones, are expected within 5 to 7 years, with wider adoption in electric aviation and transport likely within a decade.
What environmental benefits do sodium-air fuel cells offer?
They produce no greenhouse gas emissions during use, utilize abundant and non-toxic materials, and their chemical byproducts can capture atmospheric CO₂.