New Polymer Breakthrough Boosts Durability and Sustainability Using Green Chemistry: The balance between durability and sustainability in plastics has long challenged scientists and industries alike. The new polymer breakthrough that boosts durability and sustainability using green chemistry, recently developed by researchers at Osaka University, offers a groundbreaking solution to this problem. This innovative polymer maintains exceptional strength and heat resistance but, uniquely, can be recycled indefinitely without losing quality — a feat that could redefine how plastics are made, used, and recycled worldwide.
This article explores the science behind this breakthrough, its environmental and industrial impact, practical applications, and the broader context of sustainable polymers in today’s economy.
Why This Polymer Breakthrough Is a Game-Changer
Plastics are ubiquitous in modern life — from packaging and consumer goods to automotive components and electronics. However, the environmental toll of plastic waste is staggering. According to the United Nations Environment Programme, approximately 300 million tons of plastic waste is generated globally each year, with only a fraction effectively recycled. The durability that makes plastics useful often prevents efficient recycling, creating a massive environmental challenge.
This breakthrough directly addresses this contradiction by producing a polymer that is both mechanically robust and chemically recyclable. It enables the creation of high-performance plastics that can be repeatedly recycled without degradation, significantly reducing the demand for virgin materials and the volume of plastic pollution.
New Polymer Breakthrough Boosts Durability and Sustainability Using Green Chemistry
| Feature | Details |
|---|---|
| Research Institution | Osaka University (Japan) |
| Lead Researchers | Prof. Satoshi Ogawa and Prof. Mamoru Tobisu |
| Innovation Type | Durable, fully recyclable polymer enabled by green chemistry |
| Key Mechanism | Incorporation of a molecular “directing group” activated by a nickel catalyst |
| Recyclability | Depolymerization into monomers with no loss of quality |
| Environmental Impact | Supports circular plastic economy; reduces landfill and ocean pollution |
| Industrial Applications | Automotive parts, electronics housings, packaging, construction materials |
| Official Publication Link | Chemical Science Journal |
The new polymer breakthrough boosting durability and sustainability using green chemistry marks a milestone in polymer science and environmental stewardship. By enabling plastics that are both strong and infinitely recyclable, it provides industries a practical path toward reducing plastic waste and fostering a circular economy.
This advancement exemplifies how innovative chemical design can solve entrenched environmental problems without sacrificing material performance, supporting both economic growth and planetary health. As this technology moves toward commercialization, it promises to reshape how plastics are made, used, and recycled for decades to come.
What Are Polymers and Why Is This Important?
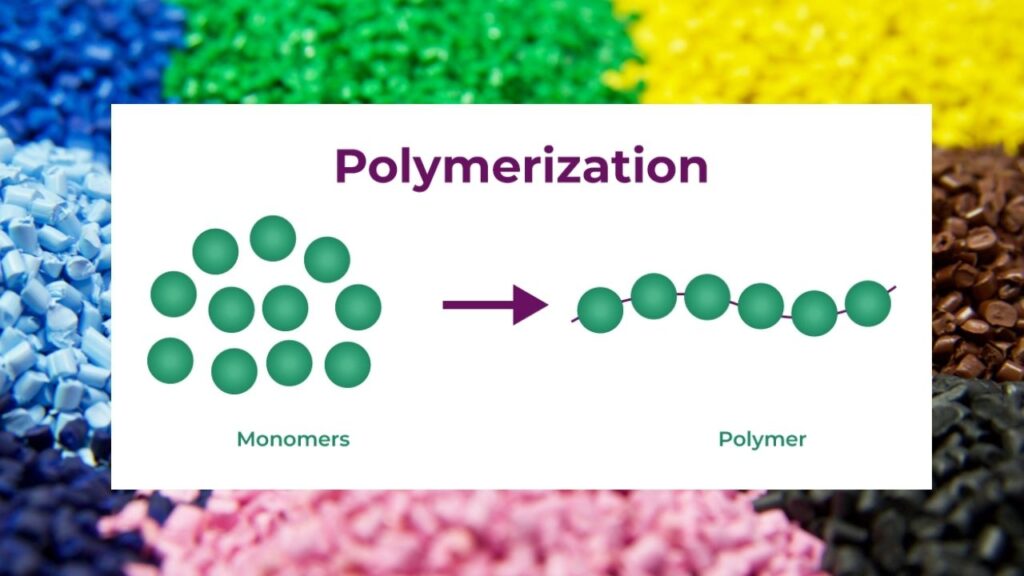
Basics of Polymers
Polymers are large molecules made up of repeated subunits called monomers. These materials are incredibly versatile and can be tailored for specific properties:
- Thermoplastics: Soften upon heating and are widely recyclable (e.g., polyethylene).
- Thermosets: Set permanently and are hard to recycle.
Most durable plastics fall into the thermoset category or are heavily crosslinked thermoplastics, making recycling difficult.
Durability Versus Recyclability: The Challenge
Durability comes from strong chemical bonds within the polymer structure. However, these same bonds make plastics resistant to breakdown during recycling. Conventional recycling often involves mechanical processes that degrade polymer chains, causing downcycling — where recycled plastics have inferior properties and limited reuse.
This dilemma results in vast amounts of plastic waste that cannot be efficiently recycled and end up polluting land and oceans.
The Science Behind the Breakthrough
The Molecular “Directing Group”
The team at Osaka University innovated by embedding a special chemical unit — a directing group — into the polymer backbone. This group serves two roles:
- Stability: Under normal use conditions, it maintains the polymer’s strength and durability.
- Recyclability Trigger: When exposed to a specific nickel catalyst, it initiates a chemical reaction that depolymerizes the polymer back into its original monomers.
How Does Depolymerization Work?
Depolymerization is the reverse of polymerization. Instead of linking monomers into long chains, the polymer chains are broken down chemically into their building blocks.
The nickel catalyst specifically interacts with the directing group, breaking the polymer bonds cleanly and selectively. Unlike traditional recycling, which uses heat or mechanical grinding, this method preserves the monomer integrity and yields material identical to virgin plastic.
Significance Compared to Current Technologies
- Mechanical Recycling: Often degrades polymer chains and yields lower-quality products.
- Chemical Recycling (General): Can break plastics down chemically but is often energy-intensive and costly.
- This New Method: Operates under mild conditions with high selectivity and efficiency, improving sustainability and economics.
Environmental Impact: A Step Toward Circular Plastic Economy
Plastic Waste Statistics
- Over 400 million tons of plastic are produced globally each year (World Bank).
- Only ~10% of plastic is effectively recycled worldwide (Our World in Data).
- Plastic pollution threatens marine biodiversity, with an estimated 8 million tons entering oceans annually (Science Advances, 2015).
How This Polymer Addresses Plastic Pollution
By enabling infinite recycling with no quality loss, this technology supports a closed-loop system where plastics are reused continuously. This can:
- Reduce reliance on fossil fuel-based virgin plastics.
- Minimize plastic waste ending in landfills and natural environments.
- Lower greenhouse gas emissions associated with plastic production and disposal.
The breakthrough aligns perfectly with green chemistry principles that emphasize waste reduction, safer chemicals, and energy efficiency.
Industrial and Economic Implications
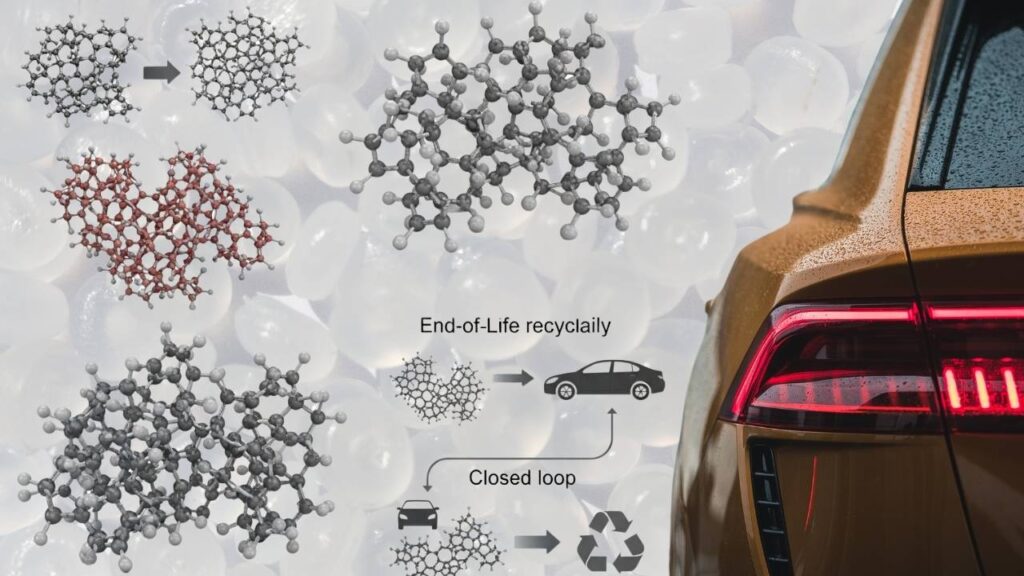
Adoption Potential in Key Sectors
- Automotive: Durable, recyclable polymers could reduce vehicle weight and emissions while supporting end-of-life recycling goals.
- Electronics: Sustainable casings that resist heat and mechanical stress but can be chemically recycled.
- Packaging: High-barrier, durable packaging materials that do not compromise recyclability.
- Construction: Long-lasting materials with recyclability reduce construction waste.
Economic Benefits
- Reduced raw material costs by reusing monomers.
- Compliance with increasing regulatory requirements globally, such as the EU’s Circular Economy Action Plan.
- Enhanced brand value by marketing sustainable products.
How Manufacturers Can Prepare to Use This Polymer Technology
Step 1: Material Assessment
Evaluate product requirements for durability, heat resistance, and recyclability. Engage with suppliers or research partners about polymers with embedded directing groups.
Step 2: Production Compatibility
Analyze if existing manufacturing processes can handle these polymers or if adjustments are needed. Since the polymer behaves like conventional durable plastics during use, transition should be manageable.
Step 3: Set Up Chemical Recycling Systems
Collaborate with chemical recyclers equipped to apply nickel catalysts for depolymerization. Consider logistics for collecting and processing end-of-life products.
Step 4: Sustainability Reporting
Implement tracking systems for recycled content and environmental impact. Communicate transparently with consumers about recyclability and lifecycle benefits.
Challenges and Future Directions
Scale-Up and Commercialization
While the chemistry is proven in labs, scaling production and recycling infrastructure for industrial volumes will require investment and collaboration across industries.
Catalyst Recovery and Cost
Developing efficient, recyclable nickel catalysts and minimizing costs remain critical for commercial viability.
Consumer Awareness and Policy Support
Encouraging adoption also depends on consumer demand for sustainable products and supportive policies that incentivize green materials.
Robotic Transfer Technique Enhances Scalability of 2D Materials for Device Integration
Child Who Received CAR-T Cancer Therapy Remains Disease-Free 18 Years Later
Quantum Computing Milestone Achieved, Immediately Challenged by Supercomputer
FAQs About New Polymer Breakthrough Boosts Durability and Sustainability Using Green Chemistry
Q1: How is this polymer different from biodegradable plastics?
A1: This polymer is not biodegradable; instead, it is chemically recyclable. Biodegradable plastics break down in the environment, which can lead to microplastic formation, while this new polymer can be recycled repeatedly without degradation.
Q2: Does the recycling process require special equipment?
A2: Yes, the chemical recycling requires a nickel catalyst and controlled chemical processes. This differs from standard mechanical recycling.
Q3: Is this technology patented or commercially licensed?
A3: The technology is published and patented by Osaka University. Commercial licensing discussions are ongoing.
Q4: Can this polymer be used in food packaging?
A4: Safety for food contact depends on further regulatory approvals and testing but the chemical purity of recycled monomers may allow it.
Q5: How does this innovation fit with global sustainability goals?
A5: It directly supports the United Nations Sustainable Development Goals (SDGs), especially SDG 12 (Responsible Consumption and Production) and SDG 13 (Climate Action).