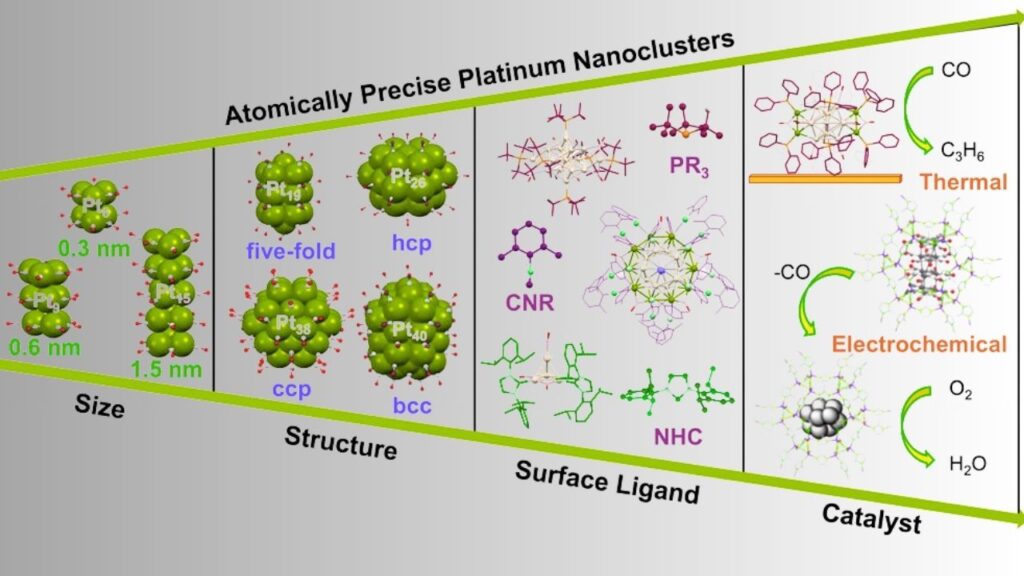
Catalytic Activity at the Sub-Nanometer Scale: In the world of chemistry and industry, new atomically precise platinum clusters are making headlines for their promising catalytic activity at the sub-nanometer scale. This breakthrough is not just for scientists in white coats—it’s a story that affects everything from how we make cleaner fuels to how we manufacture everyday products.
Let’s dive into what these platinum clusters are, why they matter, and how they could change the future of catalysis.
Catalytic Activity at the Sub-Nanometer Scale
| Aspect | Details |
|---|---|
| What’s New? | Atomically precise platinum clusters, stabilized using nanoscale confinement, show exceptional catalytic activity and stability. |
| Why It Matters | These clusters outperform both single platinum atoms and larger nanoparticles, especially in hydrogenation reactions. |
| How It Works | Clusters are “trapped” in cerium oxide nanoislands on silica, preventing them from clumping and losing efficiency. |
| Industrial Impact | Potential for more efficient, robust, and cost-effective catalysts in chemical manufacturing, energy, and environmental applications. |
| Key Stat | Clusters remain stable at temperatures up to 600°C, outperforming traditional catalysts under harsh conditions. |
| Official Resource | Nature Chemical Engineering |
New atomically precise platinum clusters are changing the game for catalysts at the sub-nanometer scale. By cleverly trapping these clusters in nanoislands, scientists have created catalysts that are more efficient, more stable, and more cost-effective than ever before. This innovation promises cleaner, greener, and more affordable chemical processes that could benefit industries, the environment, and society as a whole.
What Are Atomically Precise Platinum Clusters?
Platinum is a precious metal known for its ability to speed up (catalyze) chemical reactions. Traditionally, platinum catalysts have been used in everything from car exhaust systems to fuel cells. But scientists have discovered that when platinum is arranged in tiny clusters of just a few atoms, it can work even better than when it’s used as single atoms or larger particles.
Imagine platinum atoms as players on a soccer team. If you have just one player (a single atom), they can’t do much. If you have a huge crowd (a big nanoparticle), not everyone gets to touch the ball. But a small, well-organized team (a cluster of a few atoms) can pass, score, and win the game much more efficiently!
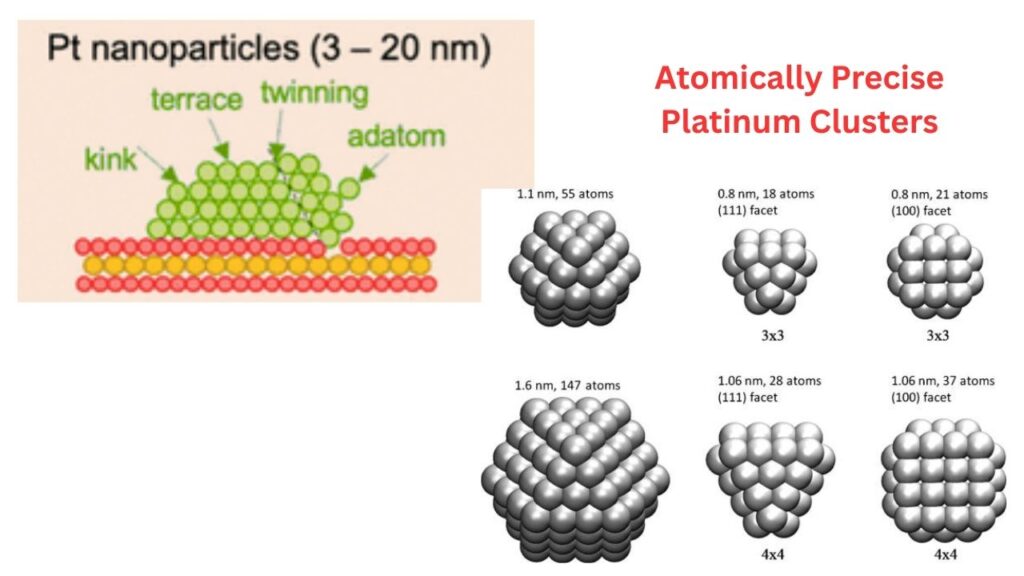
Why Are These Clusters Special?
1. Supercharged Catalytic Activity
When platinum atoms are grouped in clusters—usually between 2 and 10 atoms—they show much higher catalytic activity in certain reactions, such as hydrogenation (adding hydrogen to molecules). In fact, these clusters can outperform both single atoms and larger nanoparticles, making them the “goldilocks” of catalysts—not too big, not too small, but just right.
2. Stability Under Pressure
One big problem with tiny clusters is that they like to clump together. When they do, they lose their special properties and become less effective. But researchers have found a solution: they “trap” these clusters inside nano-sized islands of cerium oxide, which are themselves supported on silica. Each island acts like a tiny safe house, keeping the platinum clusters apart and stable—even under tough industrial conditions.
3. Efficiency and Cost Savings
Platinum is expensive and rare. By using atomically precise clusters, industries can use less platinum while getting more catalytic power, making processes cheaper and more sustainable.
How Are These Platinum Clusters Made?
Step 1: Preparing the Playground
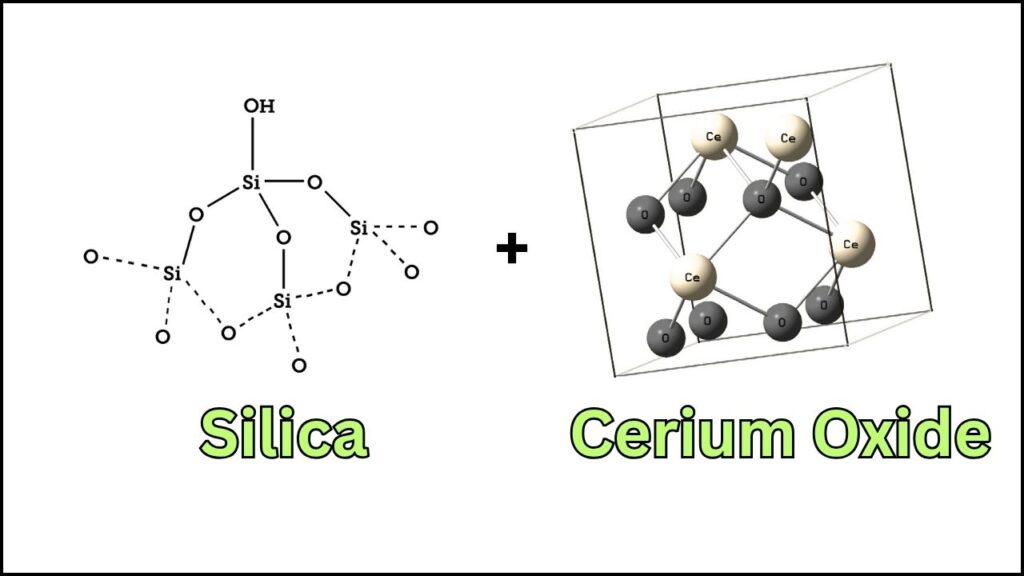
Researchers start with a special surface made of silica (a material similar to glass), decorated with tiny “islands” of cerium oxide. Think of this as a soccer field with lots of small, fenced-in areas.
Step 2: Adding Platinum Atoms
Platinum atoms are carefully placed onto these islands. The cerium oxide acts like a net, catching and holding the platinum in place.
Step 3: Heat Treatment
The whole setup is heated in a hydrogen atmosphere at around 400°C. This helps the platinum atoms gather into clusters, but the cerium oxide keeps them from wandering off and clumping together.
Step 4: Testing and Fine-Tuning
Finally, the researchers test how well these clusters work in real chemical reactions, such as turning ethylene into ethane (a common industrial process). They also use computer models to understand why certain cluster sizes work better than others.
Real-World Impact: Why Should You Care?
For Industry
- Cleaner Production: More efficient catalysts mean less waste and lower emissions.
- Cost Savings: Using less platinum reduces costs for manufacturers.
- Durability: These clusters can withstand high temperatures and harsh chemicals, making them ideal for tough industrial environments.
For the Environment
- Reduced Resource Use: Less platinum mining means less environmental impact.
- Greener Processes: More efficient reactions can lead to cleaner fuels and products.
For Science and Technology
- New Discoveries: Understanding how atoms work together at this tiny scale opens doors to new materials and technologies.
- Better Design: Scientists can now design catalysts with atomic precision, tailoring them for specific reactions.
The Science Behind the Breakthrough
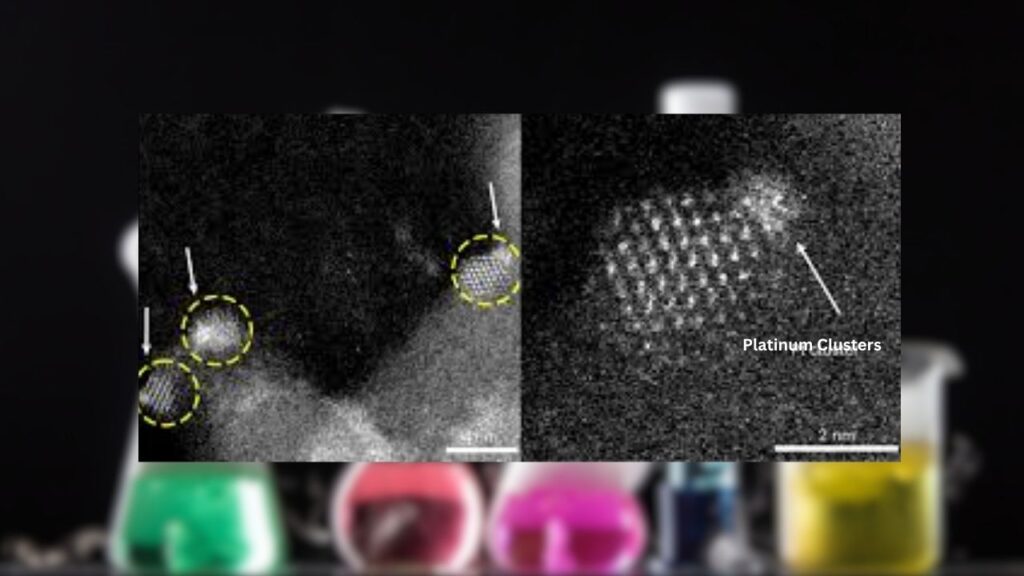
Nanoscale Confinement: The Secret Sauce
The key to this breakthrough is nanoscale confinement. By trapping the platinum clusters in tiny islands of cerium oxide, scientists prevent them from merging into bigger, less active particles. This strategy keeps the clusters stable and active, even at temperatures up to 600°C—a huge improvement over previous methods.
Optimal Cluster Size
Not all cluster sizes are created equal. The research shows that there’s an optimal size where platinum clusters are most effective. Too small, and they don’t work as well. Too big, and they lose their special properties. Finding this “sweet spot” is a big part of what makes this research so exciting.
Synergy with Cerium Oxide
Cerium oxide isn’t just a physical barrier—it also helps with the chemistry. It can store and release oxygen, and it interacts with the platinum clusters to make them even more active. This synergy is a big reason why the new catalysts work so well.
Practical Advice: How Can Industries Use This Technology?
1. Upgrade Existing Catalysts
Companies can retrofit their current catalytic systems with these new platinum clusters, improving efficiency without a complete overhaul.
2. Explore New Applications
With their stability and efficiency, these catalysts could be used in:
- Fuel cells for clean energy
- Petrochemical production
- Environmental cleanup (such as breaking down pollutants)
3. Collaborate with Researchers
Industries should partner with universities and research labs to stay at the cutting edge and help scale up these technologies for real-world use.
Simple Chemical Solution Produces Ultra-Thin Materials With Powerful Electronic Properties
Scientists Detect Mysterious Radio Pulses Coming From Deep Beneath Antarctic Ice Sheet
Optimized Solvents Significantly Improve Carrier Mobility in Metal-Organic Frameworks (MOFs)
FAQs About Catalytic Activity at the Sub-Nanometer Scale
Q1: What is a catalyst?
A catalyst is a substance that speeds up a chemical reaction without being used up itself. Platinum is a famous catalyst used in many industries.
Q2: Why does the size of platinum clusters matter?
Smaller clusters have more exposed atoms, making them more reactive. But if they get too small or too big, they lose efficiency. The right size cluster is the most effective.
Q3: How do these clusters stay stable?
They are “trapped” in nano-sized islands of cerium oxide, which keeps them from clumping together and losing their special properties.
Q4: Are these catalysts expensive?
While platinum itself is costly, using clusters means you need much less of it, which can save money in the long run.
Q5: Where can I learn more?
Check the official resource in the Key Highlights table above.