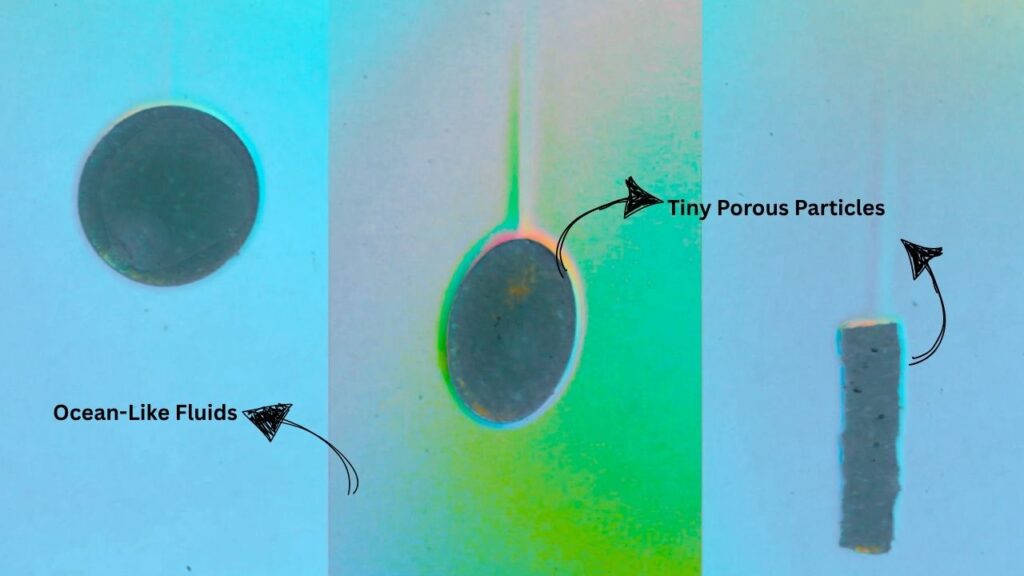
Ocean-Like Fluids Reveal Tiny Porous Particles Sink Faster: this counterintuitive finding is turning heads in the world of marine science and climate research. For decades, scientists believed that bigger particles always sink faster in water. But new research from Brown University and the University of North Carolina at Chapel Hill is rewriting the rulebook, showing that in the ocean’s layered, salty waters, small, porous particles can actually outpace their larger counterparts.
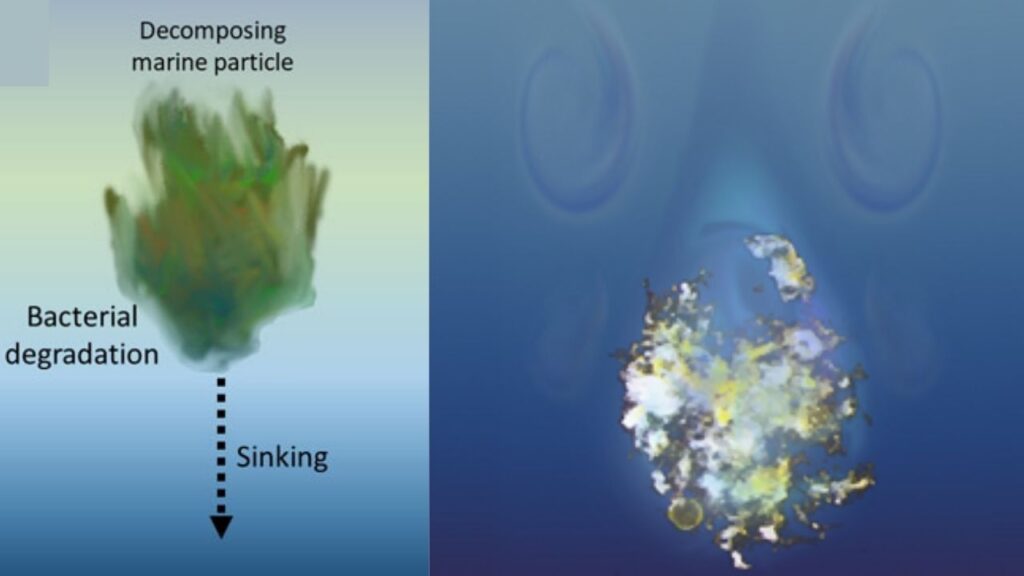
This breakthrough doesn’t just change how we think about ocean physics; it also has big implications for understanding climate change, ocean health, and even how we tackle pollution. In this article, we’ll break down the science, explain why it matters, and offer practical insights for professionals and curious minds alike.
Ocean-Like Fluids Reveal Tiny Porous Particles Sink Faster
| Key Point | Details |
|---|---|
| Discovery | Tiny porous particles can sink faster than larger ones in stratified (layered) ocean-like fluids. |
| Mechanism | Sinking speed is influenced by how much salt a particle absorbs relative to its volume, not just its size. |
| Implications | Changes understanding of carbon cycling, marine snow, and microplastic behavior in oceans. |
| Real-world Impact | Can improve climate models and inform ocean-based carbon capture strategies. |
| Official Resource | PNAS |
The discovery that tiny porous particles can sink faster than larger ones in ocean-like fluids is a game-changer for marine science. It challenges old assumptions, opens new avenues for research, and could reshape how we think about the ocean’s role in climate regulation, pollution, and carbon capture. By understanding the hidden physics of particle sinking, we gain powerful tools to protect our planet and innovate for the future.
What Are Ocean-Like Fluids and Why Do They Matter?
The ocean isn’t just a big bowl of water. It’s a layered system, with colder, saltier, and therefore denser water at the bottom, and warmer, fresher water at the top. Scientists call this a stratified fluid. These layers are crucial for everything from ocean currents to the way nutrients and carbon move around the globe.
When particles—like dead plankton, dust, or even microplastics—fall through these layers, they don’t just drop straight down. Their journey is shaped by the ocean’s changing density, temperature, and saltiness.
The Classic View: Bigger Sinks Faster
Traditionally, scientists thought that bigger particles sink faster in water. This idea comes from basic physics: gravity pulls harder on heavier objects, while water pushes back with drag. In a simple, uniform fluid, this rule holds true. That’s why, for example, a rock falls faster than a feather in water.
But the ocean is far from simple. It’s a complex, dynamic system where the rules can change.
The New Discovery: Why Tiny Porous Particles Sink Faster
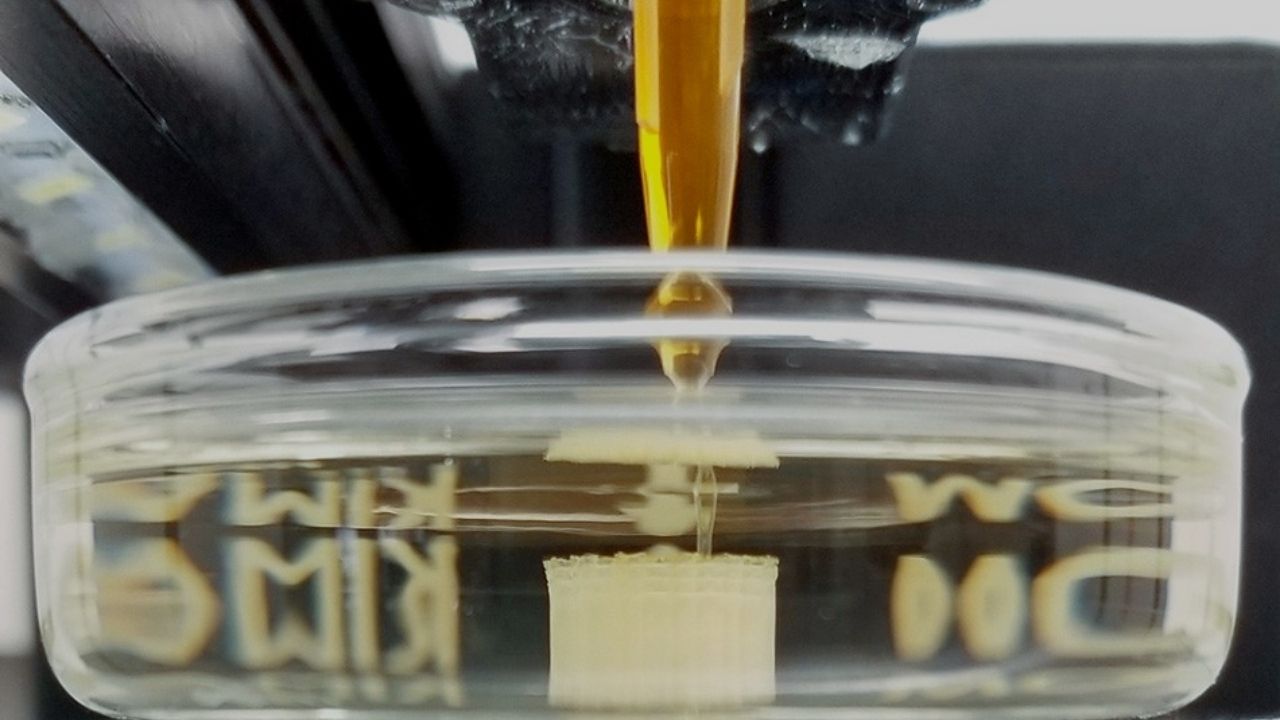
Researchers at Brown University and UNC Chapel Hill set out to test what really happens when particles sink in ocean-like, layered fluids. They used clever lab experiments with 3D-printed agar shapes—think of them as tiny, squishy models of marine snow—and watched how they moved through a tank with layers of saltwater.
Here’s what they found:
- Porosity and Salt Absorption Matter: As a particle sinks, it absorbs salt from the surrounding water. For porous (holey or sponge-like) particles, this process is much faster.
- Salt Changes Buoyancy: The more salt a particle absorbs, the heavier it gets—making it sink faster.
- Size Isn’t Everything: In stratified fluids, smaller, more porous particles can absorb salt more efficiently relative to their volume, meaning they can actually sink faster than larger, less porous ones.
- Shape Counts Too: For flat or elongated particles, the smallest dimension is key. Thin, flat particles can outpace round ones of the same volume.
“It basically means that smaller particles can sink faster than bigger ones,” said Robert Hunt, a postdoctoral researcher at Brown University. “That’s exactly the opposite of what you’d expect in a fluid that has uniform density.”
Why Does This Matter? Real-World Implications
1. Marine Snow and the Carbon Cycle
The ocean is full of “marine snow”—tiny clumps of dead plankton, fecal pellets, and dust that drift down like snowflakes. These particles are crucial for the biological carbon pump, a process that locks away carbon at the bottom of the sea. Scientists estimate that marine snow helps move 3 to 10 petagrams (Pg) of carbon per year to the deep ocean.

If small, porous particles sink faster, they’re more likely to reach the ocean floor before bacteria eat them up and turn their carbon back into CO₂. This could mean the ocean is better at storing carbon than we thought—good news for climate change.
2. Microplastics and Pollution
Microplastics are everywhere in the ocean, and many are porous. Understanding how fast they sink helps us predict where they’ll end up—on the beach, in the water, or buried in the seafloor. This knowledge can guide cleanup efforts and pollution tracking.
3. Improving Climate Models
Accurate predictions of how carbon moves through the ocean are vital for climate models. If small particles sink faster, it changes how we estimate the ocean’s ability to store carbon and regulate the Earth’s climate.
4. Ocean Engineering and Carbon Capture
These findings could help engineers design better systems for ocean-based carbon capture—technologies that aim to lock away CO₂ in the deep sea by speeding up natural processes.
How Did Scientists Make This Discovery? The Experiment Explained
The research team built a special tank with layers of saltwater, mimicking the ocean’s stratification. They dropped in 3D-printed agar particles of different shapes and sizes, then used high-speed cameras to track their movement.
What they observed:
- Small, porous particles sank faster than expected.
- Flat or elongated particles sank faster than round ones.
- The sinking speed depended on how quickly the particle absorbed salt, not just its weight or size.
They even developed a simple formula to predict sinking speed based on particle size, shape, porosity, and the ocean’s density gradient. This formula can be used by scientists and engineers to estimate how different particles will behave in real-world conditions.
Practical Advice: How Can Professionals Use This Knowledge?
For Oceanographers and Climate Scientists
- Update Models: Incorporate the new sinking speed formula into carbon cycle and nutrient transport models.
- Field Measurements: Use in situ imaging and particle tracking to validate predictions in real ocean settings.
- Collaborate: Work with physicists and engineers to refine models and explore new applications.
For Environmental Engineers
- Design Better Filters: Understanding particle behavior can help design more effective filters for microplastics and pollutants.
- Optimize Carbon Capture: Use the findings to develop materials or methods that mimic fast-sinking particles for ocean-based carbon sequestration.
For Educators and Students
- Teach the New Science: Use this discovery as a real-world example of how science evolves and why questioning assumptions leads to breakthroughs.
- Hands-On Experiments: Simple tank experiments with saltwater and different materials can demonstrate these principles in the classroom.
Quantum‑Si to Participate in GenomeWeb Webinar on Proteomics Data Standardization
James Webb Telescope Data Suggest Universe Might Be Inside a Huge Black Hole
FAQs About Ocean-Like Fluids Reveal Tiny Porous Particles Sink Faster
Q1: Why do particles absorb salt as they sink?
A: Porous particles act like sponges, soaking up salt from the surrounding water. This increases their density, making them sink faster in stratified fluids.
Q2: Does this mean all small particles sink faster than big ones?
A: Not always. The effect depends on the particle’s porosity and the ocean’s density layers. In uniform fluids, bigger still sinks faster. In layered fluids, porosity and salt absorption can flip the script.
Q3: How fast do marine snow particles actually sink?
A: Sinking speeds vary widely—from a few meters to several hundred meters per day—depending on size, shape, porosity, and ocean conditions.
Q4: How does this affect climate change predictions?
A: If more carbon-rich particles reach the deep ocean, the ocean may store more carbon than previously thought, potentially slowing climate change.
Q5: Where can I read the original research?
A: The study is published in the Proceedings of the National Academy of Sciences. For more details, visit the official website (see Key Highlights table).