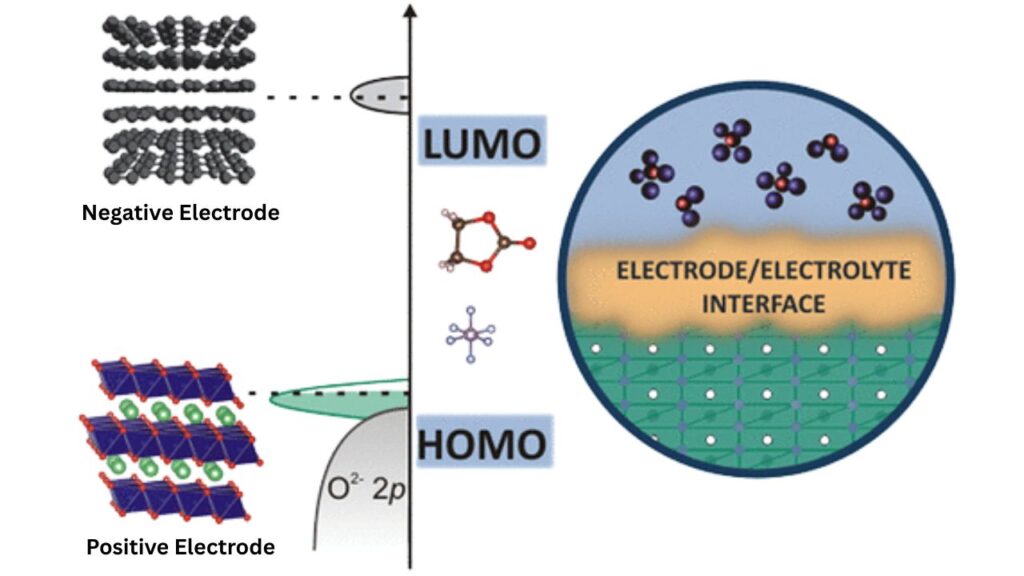
Heterogeneities at Electrode–Electrolyte Interfaces in Li Batteries: Lithium batteries power everything from smartphones to electric cars, and at the heart of these energy storage devices lies a complex dance between the electrode and the electrolyte. The place where they meet—the electrode–electrolyte interface—is a hotbed of scientific intrigue. One of the biggest challenges facing battery researchers today is understanding and controlling the heterogeneities at these interfaces. These tiny differences can make or break a battery’s performance, safety, and lifespan.
In this article, we’ll break down what these heterogeneities are, why they matter, and what the latest research reveals. Whether you’re a curious student, a battery engineer, or just someone who wants their phone to last longer, this guide will help you understand this crucial topic.
Heterogeneities at Electrode–Electrolyte Interfaces in Li Batteries
| Topic | Details |
|---|---|
| Definition | Heterogeneities are variations in chemistry, structure, or concentration at the electrode–electrolyte interface. |
| Why Important | They affect battery safety, performance, and lifespan. |
| Key Findings | Concentration and chemical heterogeneities can cause phase separation, leading to battery degradation. |
| Recent Research | Advanced imaging and molecular studies reveal the impact of these heterogeneities on lithium transport and interface stability. |
| Practical Advice | Interface engineering and electrolyte additives can help mitigate negative effects. |
| Career Insight | Materials scientists, electrochemists, and engineers are in high demand for battery research. |
| Official Resource | Nature Portfolio – Example Study |
Heterogeneities at electrode–electrolyte interfaces in lithium batteries are a critical factor that can determine whether your battery lasts for years or fails in months. By understanding what causes these differences and how to control them, scientists and engineers are paving the way for safer, longer-lasting, and more powerful batteries. The future of energy storage depends on mastering this tiny, but mighty, interface.
What Are Heterogeneities at Electrode–Electrolyte Interfaces?
Heterogeneities are simply differences or irregularities that occur at the microscopic or even atomic level where the electrode and electrolyte meet inside a lithium battery. Imagine a road with smooth and rough patches—cars (or in this case, lithium ions) will travel differently depending on the surface. In batteries, these “patches” can be:
- Chemical: Different materials or compounds forming in certain spots.
- Structural: Variations in how molecules or crystals are arranged.
- Concentration: Areas with more or less lithium or other ions.
These differences are not just academic—they directly affect how well a battery works. For example, if lithium ions can’t move smoothly across the interface, the battery may lose capacity or even fail.
Why Do These Heterogeneities Matter?
1. Battery Performance
When the interface is uneven, lithium ions may pile up in certain spots, leading to nonuniform lithium plating. This can cause dangerous spikes in current, reduce energy storage, and shorten battery life.
2. Safety Risks
Uneven interfaces can trigger the growth of lithium dendrites—tiny, needle-like structures that can pierce through the battery and cause short circuits or fires. This is a major concern in high-energy applications like electric vehicles.
3. Lifespan and Reliability
Heterogeneities can lead to the formation of unstable layers, such as the solid electrolyte interphase (SEI), which may degrade over time and increase resistance, making the battery less efficient.
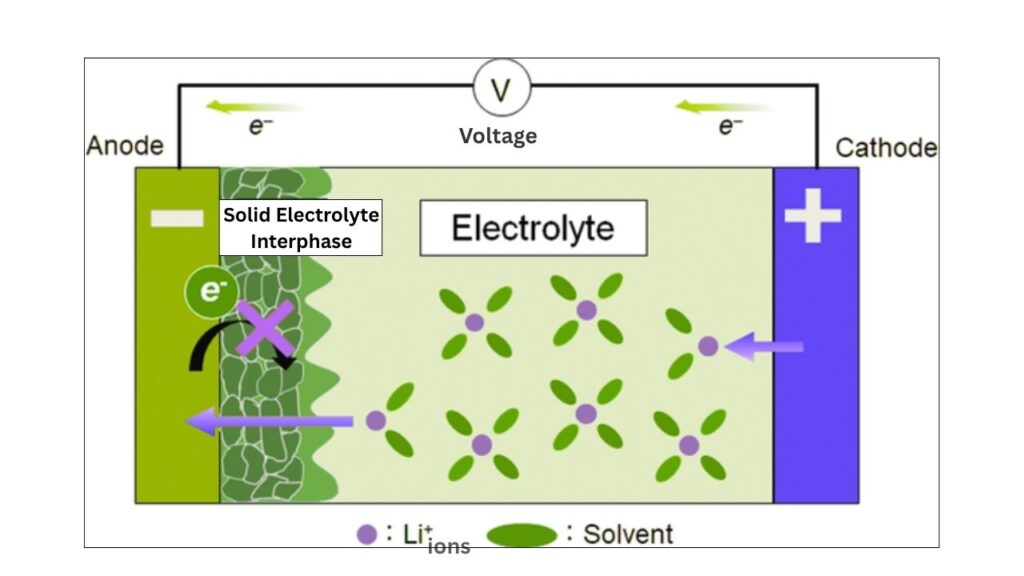
How Do Heterogeneities Form?
A. Chemical and Concentration Heterogeneities
- Electrolyte Decomposition: During charging and discharging, the electrolyte can break down unevenly, leading to patches of different chemical compounds at the interface.
- Phase Separation: In polymer electrolytes, certain regions may lose their ability to conduct ions, causing a separation into “mobile” and “rigid” phases.
B. Structural Heterogeneities
- Molecular Arrangement: The way polymer chains or inorganic crystals are packed can vary, affecting how easily lithium ions move.
- Void Formation: Gaps or voids can appear at the interface, especially under low pressure, disrupting the contact between electrode and electrolyte.
C. Spatial Heterogeneities
- Surface Roughness: Imperfections on the electrode surface can concentrate current in certain areas, making problems worse.
- Impurities: Tiny bits of unwanted material can create “hot spots” where reactions happen faster or slower than normal.
Real-World Example: Polymer Electrolyte Interfaces
Recent studies using advanced X-ray and electron microscopy have shown that in multiphase polymer electrolytes (which have both flexible and rigid parts), the interface with the electrode can become depleted of the mobile, ion-conducting phase. This leads to:
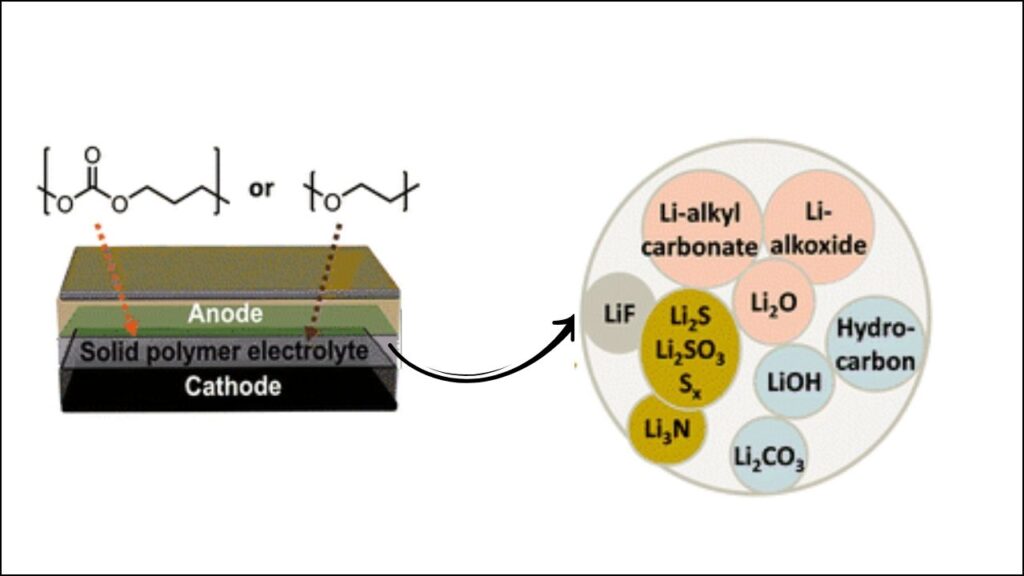
- Phase separation at the interface,
- Reduced lithium transport,
- Increased resistance and faster battery degradation.
Researchers have visualized these heterogeneities and are developing strategies—like adding special molecules (additives)—to make the interface more uniform and stable.
How Do Scientists Study These Interfaces?
Because the interface is buried deep inside the battery and only nanometers thick, it’s tough to study. Scientists use:
- X-ray synchrotron measurements to see chemical and concentration differences.
- Operando electron microscopy to watch voids and lithium movement in real time.
- Atomic-scale simulations to understand how electrons move across different materials in the SEI.
These tools help researchers design better batteries by revealing what’s really happening at the interface.
Practical Advice: How Can We Reduce Heterogeneities?
1. Interface Engineering
Carefully designing the electrode and electrolyte materials can help create a more uniform interface. For example, using molecular ionic composites or adding specific additives can improve stability.
2. Material Purity
Using high-purity materials reduces the chances of unwanted reactions and impurities that cause heterogeneities.
3. Advanced Electrolyte Design
Developing new types of electrolytes—like those with both rigid and flexible parts—can help maintain good contact and ion transport at the interface.
4. Pressure Control
Applying the right amount of pressure during battery assembly can help maintain intimate contact and prevent voids.
Step-by-Step Guide: Understanding and Managing Heterogeneities
Step 1: Identify the Interface
Know where the electrode and electrolyte meet. This is where most problems start.
Step 2: Characterize the Interface
Use advanced imaging and chemical analysis to spot differences in composition, structure, and concentration.
Step 3: Analyze the Impact
Determine how these heterogeneities affect lithium movement, battery resistance, and overall performance.
Step 4: Develop Solutions
Try different materials, additives, and assembly techniques to reduce heterogeneities.
Step 5: Test and Iterate
Build prototype batteries, test them under real-world conditions, and refine your approach based on results.
Scientists Uncover Rare Brain Activity That Could Unlock New Frontiers in Human Consciousness
Photonic Nanofabrication Enables Miniaturized Neutral Atom Quantum Networking
DNA Enabled Nanostructures Proposed To Achieve Superradiance For Quantum Light Sources
FAQs About Heterogeneities at Electrode–Electrolyte Interfaces in Li Batteries
Q1: What causes heterogeneities at the electrode–electrolyte interface?
A1: They are caused by uneven chemical reactions, phase separation, impurities, and differences in material structure at the microscopic level.
Q2: Why are these heterogeneities a problem?
A2: They can lead to uneven lithium movement, dendrite growth, increased resistance, and faster battery degradation.
Q3: Can these issues be fixed?
A3: Yes, through interface engineering, material selection, and advanced manufacturing techniques, researchers are finding ways to reduce heterogeneities and improve battery performance.
Q4: Who works on these problems?
A4: Materials scientists, electrochemists, and engineers in academia and industry are at the forefront of this research.
Q5: Where can I learn more?
A5: Refer to the official resource in the Key Highlights table above for a detailed scientific study.