Imagine a world where sunlight transforms greenhouse gases into valuable products, helping the environment and powering industries. This vision is closer to reality thanks to a breakthrough in photocatalysis: tiny molybdenum clusters on TiO₂ nanosheets. These minuscule structures are changing how scientists approach clean energy, chemical manufacturing, and pollution control.
In this article, you’ll discover what makes this innovation so exciting, how it works, and why it matters for both our planet and future technologies. Whether you’re a curious student, a professional researcher, or someone passionate about green solutions, you’ll find practical knowledge and inspiring possibilities here.
Tiny Molybdenum Clusters on TiO₂ Nanosheets Could Revolutionize Photocatalysis
| Feature/Metric | Details & Data |
|---|---|
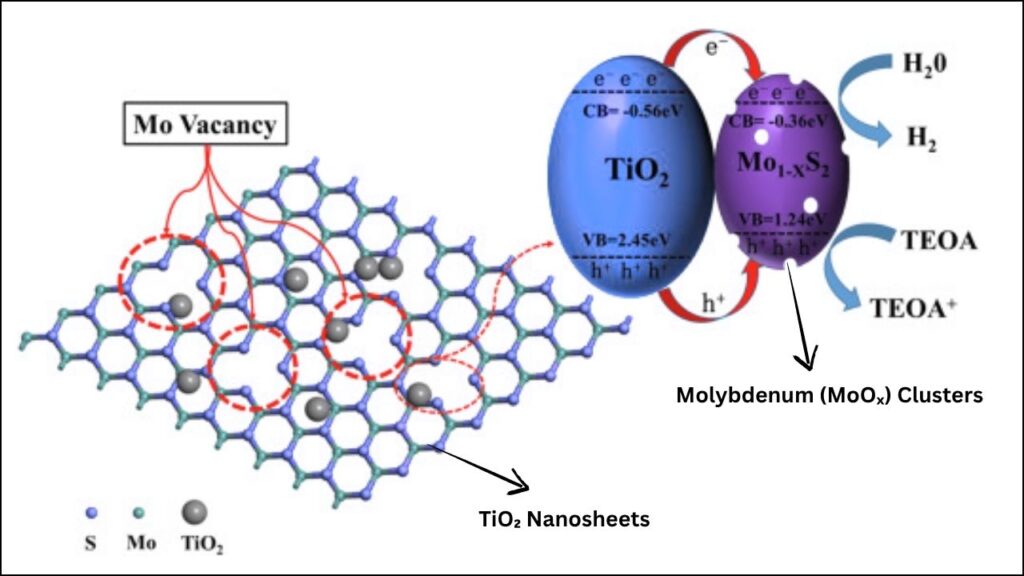
| Photocatalyst | TiO₂ nanosheets with subnanometric MoOₓ clusters (size: ~0.6 nm) |
| Target Reaction | Selective oxidation of methane to methanol and other oxygenates |
| Selectivity | Nearly 100% selectivity for organic oxygenates, minimal CO₂ formation |
| Yield | 3.8 mmol/g organic oxygenates in 2 hours (with 0.5% MoOₓ loading) |
| Quantum Yield | 13.3% at 365 nm, indicating high solar energy utilization |
| Stability | Maintained >95% selectivity over 1,800 minutes of continuous operation |
| Mechanism | Enhanced charge separation, suppressed overoxidation, S-scheme heterojunction formation |
The discovery of tiny molybdenum clusters on TiO₂ nanosheets is a game-changer for photocatalysis and green chemistry. By enabling the clean, selective, and solar-powered conversion of methane into valuable chemicals, this innovation addresses urgent environmental and industrial needs. As research continues and technology matures, we may soon see sunlight-powered factories turning greenhouse gases into the building blocks of our modern world—cleanly, efficiently, and sustainably.
Understanding the Building Blocks: TiO₂ Nanosheets and Molybdenum Clusters
What Is Titanium Dioxide (TiO₂)?
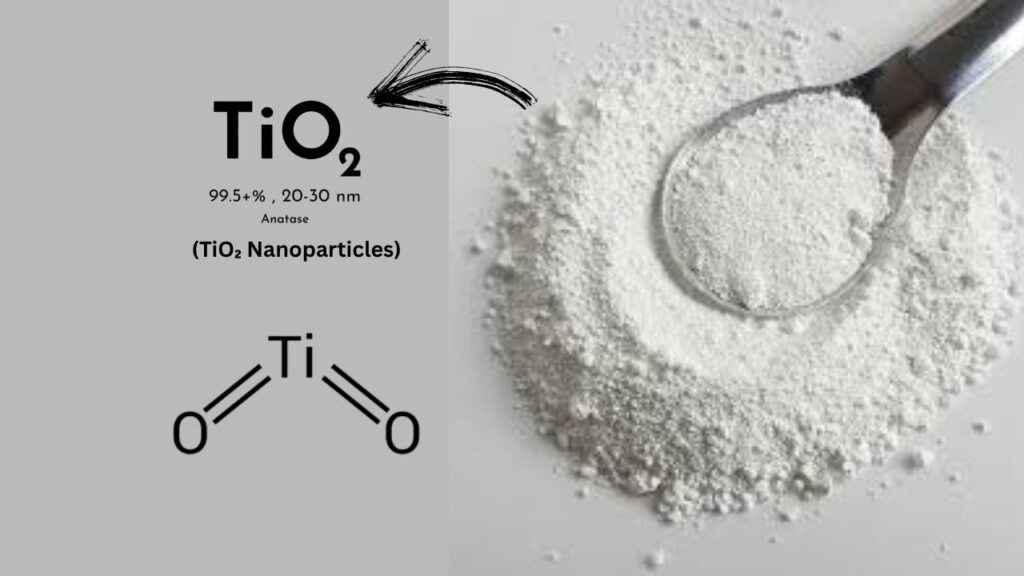
Titanium dioxide, often called TiO₂, is a white mineral used in paints, sunscreens, and even toothpaste. But when scientists engineer it into nanosheets—ultra-thin sheets just a few atoms thick—it becomes much more than a simple pigment. These nanosheets have a huge surface area and can absorb light efficiently, making them perfect for photocatalysis—the process of using light to speed up chemical reactions.
What Are Molybdenum Clusters?
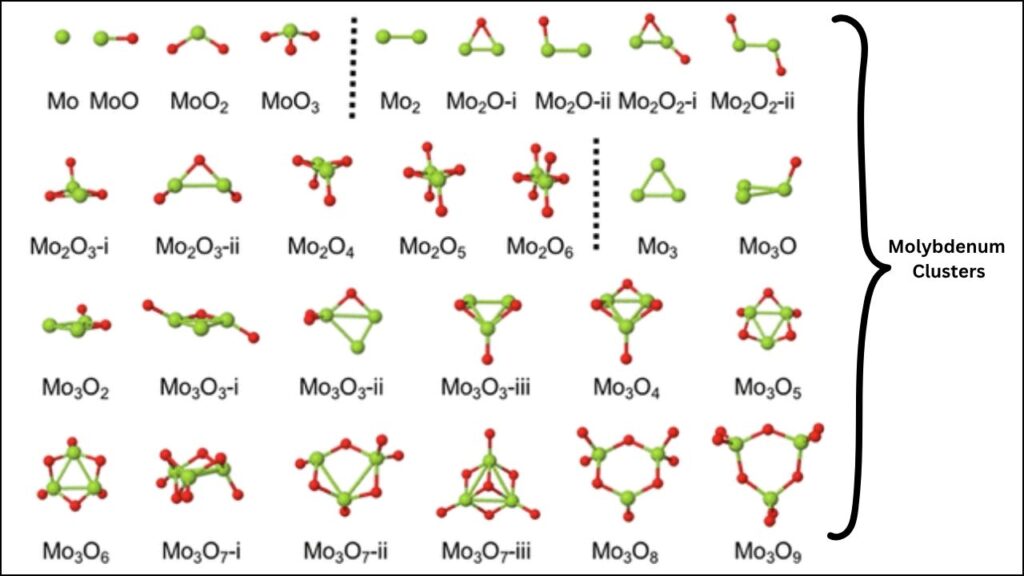
Molybdenum oxide clusters are groups of molybdenum and oxygen atoms bonded together. When these clusters are subnanometric (smaller than one nanometer), they behave differently from larger particles. Their tiny size means they have unique electronic properties and can act as super-efficient reaction sites.
Why Combine Them?
When tiny molybdenum clusters are anchored onto TiO₂ nanosheets, the result is a new kind of catalyst that’s far more powerful and selective than either material alone. This combination is like pairing a solar panel with a super-smart control system: the TiO₂ captures sunlight, and the molybdenum clusters direct the energy to do useful work.
The Methane Problem: Why This Breakthrough Matters
Methane’s Double-Edged Sword
Methane is a major component of natural gas and an extremely potent greenhouse gas—over 25 times more effective at trapping heat in the atmosphere than carbon dioxide over a 100-year period. While methane is a valuable fuel, it’s also a significant contributor to climate change when released into the air.
The Challenge of Selective Oxidation
Turning methane into useful chemicals like methanol is a top priority for scientists. Methanol is a versatile fuel and a building block for plastics, pharmaceuticals, and more. However, methane’s strong chemical bonds make it tough to convert without producing unwanted byproducts, especially carbon dioxide.
Traditional catalysts often over-oxidize methane, turning it into CO₂ instead of stopping at methanol or other valuable “oxygenates.” This wastes energy and undermines environmental goals.
How Tiny Molybdenum Clusters on TiO₂ Nanosheets Work
Step 1: Harnessing Sunlight
The process starts when sunlight shines on the TiO₂ nanosheets. The energy from the light excites electrons in the material, creating pairs of negatively charged electrons and positively charged “holes.” These charges are the driving force behind chemical reactions on the catalyst’s surface.
Step 2: Charge Separation and Transfer
Here’s where the tiny molybdenum clusters come in. They act as specialized sites that help keep the electrons and holes apart, preventing them from simply recombining and wasting their energy. This separation is crucial for making the catalyst efficient.
Step 3: Activating Methane
Methane molecules land on the surface of the catalyst. The separated charges from the TiO₂ and MoOₓ clusters work together to break the tough C-H bonds in methane, starting the process of turning it into methanol or other oxygen-rich chemicals.
Step 4: Guiding the Reaction
The molybdenum clusters don’t just help break bonds—they also guide the chemical pathway so that methane is converted into valuable products, not just burned to CO₂. This is called selective oxidation. The clusters create a unique environment that favors the formation of methanol and formaldehyde, with almost no waste.
Step 5: Collecting the Products
The resulting chemicals—primarily methanol and other oxygenates—can be collected and used for fuels, manufacturing, or other applications. The process is powered by sunlight and doesn’t require expensive or rare metals.
Why Is This Discovery So Impactful?
Record-Breaking Selectivity
One of the most impressive achievements of this catalyst is its nearly 100% selectivity for turning methane into useful oxygenates, with almost no carbon dioxide produced. This level of precision is rare in chemical catalysis and is a major step forward for green chemistry.
High Yield and Efficiency
With a yield of 3.8 millimoles of product per gram of catalyst in just two hours and a quantum yield of 13.3% at 365 nm, this system is highly efficient. Quantum yield measures how effectively the catalyst converts photons (light particles) into chemical products—a higher number means more efficient use of sunlight.
Long-Lasting Stability
The catalyst keeps working for a long time, maintaining its high selectivity and efficiency for over 1,800 minutes (that’s 30 hours!) of continuous operation. This durability is essential for real-world industrial applications.
Real-World Applications and Future Potential
Cleaner Chemical Manufacturing
Methanol is used to make everything from fuels to plastics. Using sunlight and methane to create methanol in a clean, efficient way could revolutionize the chemical industry, making it greener and less dependent on fossil fuels.
Reducing Greenhouse Gas Emissions
By converting methane—a greenhouse gas—into valuable products instead of letting it escape or burning it, this technology helps tackle climate change from two directions: reducing emissions and providing sustainable alternatives to traditional fuels.
Unlocking Stranded Gas Resources
Many oil and gas fields have “stranded” methane that’s too expensive or difficult to transport. This technology could turn that wasted gas into valuable chemicals right at the source, reducing flaring and improving resource efficiency.
Versatility for Other Reactions
While methane-to-methanol is a headline application, the principles behind this catalyst could be adapted for other important reactions, such as breaking down pollutants or producing hydrogen fuel from water.
Practical Guide: How to Use and Optimize These Catalysts
For Researchers
- Synthesis: Creating TiO₂ nanosheets with anchored MoOₓ clusters requires precise control over temperature, concentration, and deposition techniques. Methods like impregnation, sol-gel, or atomic layer deposition can be used.
- Optimization: The best performance is seen with about 0.5% molybdenum oxide by weight. Too much or too little can reduce selectivity or efficiency.
- Testing: Use controlled light sources and gas mixtures to monitor product yields and selectivity over time.
For Industry
- Scalability: While the technology is promising in the lab, scaling up will require engineering solutions for large-scale synthesis and reactor design.
- Integration: These catalysts could be integrated into existing chemical plants or used in modular units for remote or stranded gas sites.
- Sustainability: The use of earth-abundant materials (titanium and molybdenum) means the process is more sustainable and less reliant on expensive, rare metals.
Nanotechnology Unveiled: Tiny Innovations That Could Solve Huge Problems
MicroscopyGPT Uses AI to Describe Atomic Structures in 2D Materials with Unmatched Precision
Nanotech-Enhanced Fertilizer Promises More Efficient Farming With Less Environmental Damage
FAQs About Tiny Molybdenum Clusters on TiO₂ Nanosheets Could Revolutionize Photocatalysis
What makes these catalysts different from traditional ones?
Traditional catalysts often use expensive noble metals and struggle to control the reaction, leading to wasteful byproducts. The combination of TiO₂ nanosheets and tiny molybdenum clusters is more selective, efficient, and sustainable.
Can this technology be used with other greenhouse gases?
While this breakthrough focuses on methane, the principles could be adapted for other gases, such as carbon dioxide, to create valuable products and reduce emissions.
Is the process safe and environmentally friendly?
Yes. The process uses sunlight as the energy source, does not require toxic chemicals, and minimizes waste byproducts. It’s a model for green chemistry.
How soon could we see this technology in use?
While results are promising, commercial adoption will depend on further research, cost analysis, and engineering development. Pilot projects could emerge within the next decade.
What are the main challenges ahead?
Scaling up the synthesis of these catalysts, designing reactors for industrial use, and ensuring long-term stability under real-world conditions are key challenges for researchers and engineers.