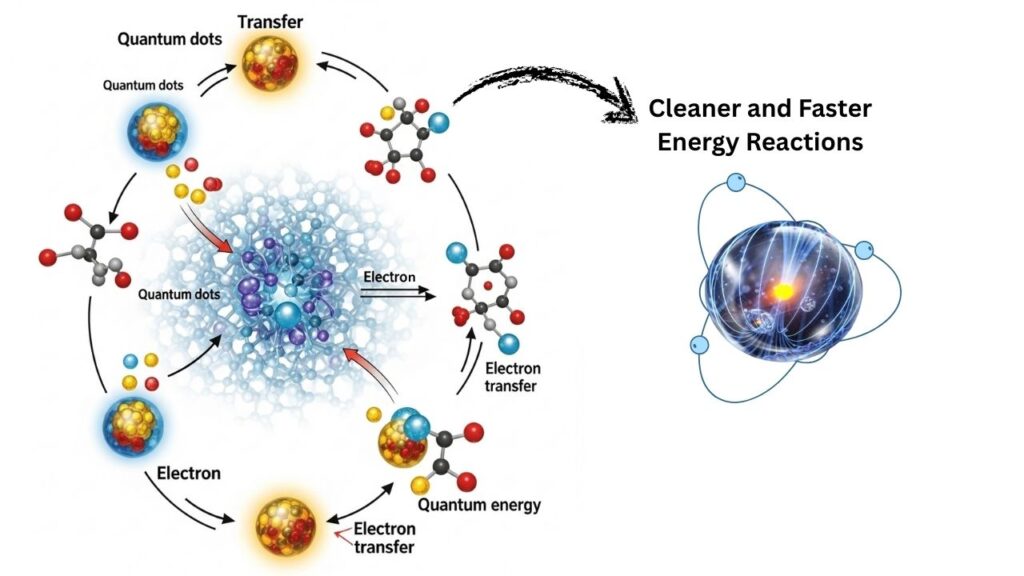
Quantum Dots Unlock Cleaner, Faster Energy Reactions—this isn’t just a scientific headline, but a glimpse into a future where energy is cleaner, chemical manufacturing is safer, and our environment is better protected. In a major scientific advance, researchers have discovered how quantum dots—tiny, engineered crystals—can transform the way we use light to power important chemical reactions. This breakthrough could pave the way for greener factories, more efficient solar panels, and even new ways to clean up pollution.
What Are Quantum Dots?
Quantum dots are incredibly small particles—thousands of times smaller than the width of a human hair. They’re made from semiconductor materials, which means they can conduct electricity under certain conditions. What makes quantum dots special is their ability to absorb and emit light in unique ways. When exposed to light, they can release energy with remarkable efficiency. Because of their size, quantum dots behave differently from larger materials, giving them properties that scientists can “tune” by changing their composition or size.
These properties have already made quantum dots useful in TV screens, medical imaging, and solar cells. But their greatest potential may be in photocatalysis—using light to speed up chemical reactions.
Why Is This Breakthrough Important?
Traditional methods for powering chemical reactions with light often require a lot of energy, expensive materials, and sometimes produce unwanted byproducts. Quantum dots offer a new path forward:
- Efficiency: The new quantum dot system uses up to 99% less light energy for certain reactions, making it much more energy-efficient.
- Versatility: These quantum dots can drive a wide range of reactions, including breaking some of the toughest chemical bonds in organic molecules.
- Cleaner Reactions: By relying on visible light and avoiding harsh chemicals, these reactions generate less pollution and are safer for people and the planet.
- Speed: Thanks to a unique mechanism, quantum dots can make reactions happen much faster than traditional catalysts.
Quantum Dots Unlock Cleaner, Faster Energy Reactions
| Aspect | Details |
|---|---|
| Breakthrough | Quantum dots as ultra-efficient photocatalysts for energy and organic reactions |
| Energy Savings | New QD system uses up to 99% less light energy than traditional methods |
| Key Mechanism | Two-photon spin-exchange Auger process in Mn²⁺-doped CdS/ZnS quantum dots |
| Applications | Birch reduction, breaking strong chemical bonds, hydrogen production, green chemistry |
| Reduction Potential | Can handle reactions with reduction potentials as low as −3.4 V (Vs. SCE) |
| Professional Impact | Opens new avenues for sustainable chemical manufacturing and renewable energy |
| Official Source | HKUST School of Science |
The discovery that quantum dots can unlock cleaner, faster energy reactions is a milestone for chemistry, energy, and environmental science. By using up to 99% less energy and enabling reactions that were previously impossible or impractical, quantum dots are paving the way for a more sustainable and efficient future. Whether you’re a scientist, engineer, student, or simply someone interested in the future of technology, this is a breakthrough worth following closely.
How Do Quantum Dots Work as Photocatalysts?
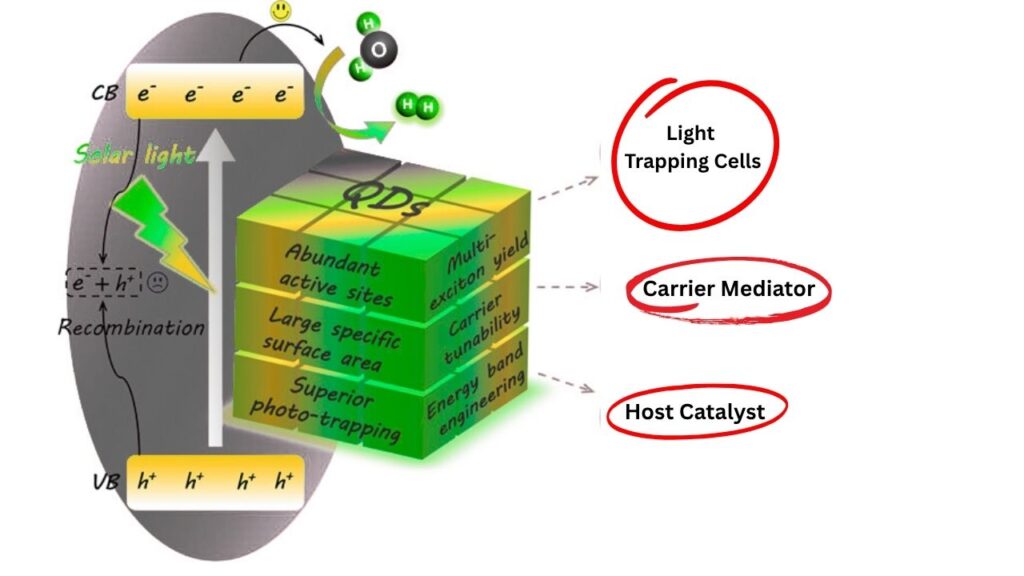
Let’s break down the science in a way that’s easy to understand:
1. Light Absorption
Quantum dots are like tiny solar panels. When you shine light on them, they absorb the energy and their electrons get “excited”—jumping to higher energy levels.
2. Hot-Electron Generation
The real magic happens in the new quantum dot system developed by researchers. They designed quantum dots with a special recipe: manganese-doped cadmium sulfide/zinc sulfide (Mn²⁺-doped CdS/ZnS). These dots use a process called the two-photon spin-exchange Auger process. This means the quantum dots can create “hot electrons”—electrons with a lot of energy—more efficiently than ever before.
3. Energy Transfer
These hot electrons are quickly transferred to the molecules involved in a chemical reaction. Because the transfer happens so fast, very little energy is lost as heat.
4. Reaction Completion
The chemical reaction finishes, often using much less energy and producing fewer byproducts than traditional methods. This is especially important for reactions that normally require strong chemicals or high temperatures.
Real-World Applications: Transforming Industries
Organic Synthesis
Chemists often need to break strong chemical bonds to build new medicines, plastics, or specialty chemicals. The new quantum dot photocatalyst can perform these reactions under mild conditions, making the process safer, less expensive, and more environmentally friendly.
Example: The Birch Reduction
The Birch reduction is a classic chemical reaction that modifies aromatic rings in organic molecules. Traditionally, it requires harsh chemicals and extreme conditions. With quantum dots, this reaction can now be performed using visible light and far less energy, making it accessible for greener manufacturing.
Hydrogen Production
Hydrogen is a clean fuel, but producing it efficiently has always been a challenge. Quantum dots can absorb sunlight and use that energy to split water molecules, releasing hydrogen gas. This process, called photocatalytic hydrogen evolution, is a major step toward using solar energy to power our world.
Environmental Cleanup
Quantum dots can help break down pollutants in water or air using only sunlight. For example, they can degrade harmful dyes in wastewater or break down pesticides, offering new solutions for cleaning up the environment.
Solar Energy Conversion
Because quantum dots can absorb a wide range of light wavelengths and convert them into usable energy, they are being explored for use in next-generation solar panels that are more efficient and flexible than traditional silicon-based panels.
The Science Behind the Breakthrough
The key innovation lies in the use of Mn²⁺-doped CdS/ZnS quantum dots and the discovery of the two-photon spin-exchange Auger process. Here’s why this matters:
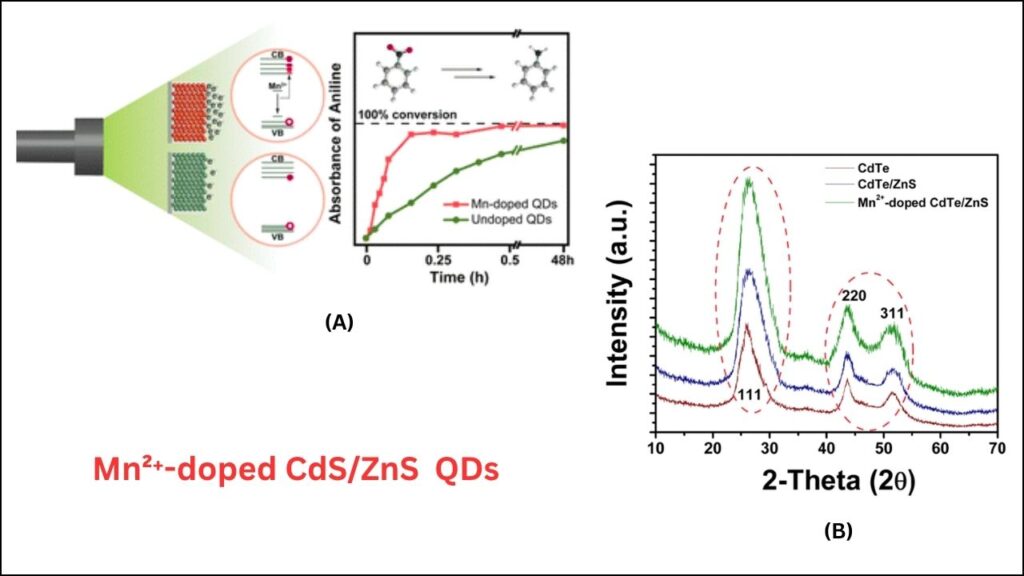
- Reduction Potentials: The new system can drive reactions with reduction potentials as low as −3.4 V (Vs. SCE), meaning it can break some of the strongest chemical bonds found in organic molecules.
- Energy Efficiency: By capturing energy before it’s lost as heat, the process is significantly more efficient than traditional photocatalysts.
- Selectivity: Quantum dots can be engineered to target specific reactions, reducing unwanted side products.
How This Breakthrough Could Impact Your Work
For Chemists and Chemical Engineers
- New Synthetic Pathways: Quantum dots open up new ways to make complex molecules, potentially reducing costs and environmental impact.
- Scalability: Their colloidal nature means quantum dots can be used in large-scale reactors, making them suitable for industrial applications.
- Safer Processes: The ability to use visible light and avoid harsh chemicals improves workplace safety.
For Renewable Energy Professionals
- Better Solar Cells: Quantum dot-based solar panels could offer higher efficiency and flexibility.
- Hydrogen Economy: Efficient hydrogen production using quantum dots could accelerate the transition to clean fuels.
- Lower Costs: Reduced energy requirements mean lower operational costs for energy production.
For Environmental Scientists
- Pollution Control: Quantum dots can break down pollutants using only sunlight, offering new tools for environmental cleanup.
- Sustainable Remediation: The technology supports eco-friendly methods for treating contaminated water and soil.
For Students and Educators
- Learning Opportunity: Quantum dots are a perfect example of how nanotechnology and chemistry can work together to solve real-world problems. They’re also a great topic for science projects and classroom discussions.
- Career Pathways: As quantum dot technology grows, so will the demand for skilled professionals in chemistry, materials science, and environmental engineering.
Practical Guide: How Quantum Dots Could Shape the Future
Step 1: Understanding Quantum Dot Synthesis
Quantum dots are synthesized using chemical methods that allow precise control over their size and composition. Researchers are continually developing safer, more sustainable ways to produce quantum dots, including using less toxic materials.
Step 2: Integrating Quantum Dots into Photocatalytic Systems
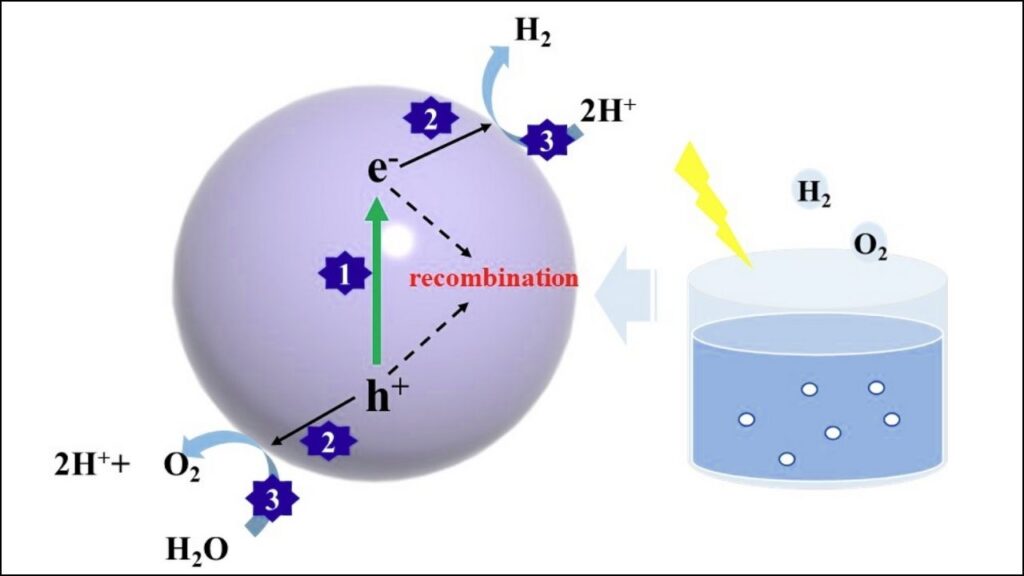
Once synthesized, quantum dots can be integrated into reactors or coatings. Their colloidal (liquid) form makes them easy to mix with other chemicals or spread on surfaces, allowing for flexible applications.
Step 3: Optimizing Light Sources
Quantum dots work best with visible light, which is abundant and inexpensive. This means they can be powered by sunlight or energy-efficient LEDs, reducing the need for specialized equipment.
Step 4: Scaling Up for Industry
Because quantum dots are stable and can be produced in large quantities, they are suitable for industrial-scale applications. This scalability is key for widespread adoption in manufacturing, energy, and environmental sectors.
Step 5: Monitoring and Safety
As with any new technology, it’s important to monitor the environmental and health impacts of quantum dots. Researchers are developing new formulations that avoid heavy metals and minimize risks, ensuring that the benefits outweigh any potential downsides.
Quantum Dots Power Next-Gen Displays: What to Expect from Future Screens
Canada Advances Quantum Error Correction Techniques for Scalable Quantum Computing
Graphene Breakthrough Promises Faster, Smaller, More Efficient Electronics
FAQs About Quantum Dots Unlock Cleaner, Faster Energy Reactions
Q: What are quantum dots made of?
A: Quantum dots are typically made from semiconductor materials such as cadmium selenide (CdSe), cadmium sulfide (CdS), or newer, less toxic materials like zinc sulfide (ZnS) and silicon.
Q: Are quantum dots safe?
A: While early quantum dots contained heavy metals, newer versions are being developed to be more environmentally friendly and less toxic. Ongoing research is focused on ensuring their safety for people and the environment.
Q: How do quantum dots compare to traditional photocatalysts?
A: Quantum dots offer higher efficiency, tunability, and can work under milder conditions, making them superior for many applications.
Q: Can quantum dots be used in everyday products?
A: Yes! They are already used in some TV screens and are being developed for use in solar panels, sensors, and medical imaging.