New eco-friendly method creates giant fullerenes cheaply—this game-changing innovation is capturing the imagination of scientists and industry experts worldwide. Imagine building tomorrow’s electronics, sustainable energy devices, smart coatings, and targeted drug delivery systems, all using advanced carbon nanostructures produced safely, affordably, and with minimal environmental burden. Thanks to the dedication and vision of research teams in Brazil and Italy, this dream is on the verge of becoming everyday reality.

Let’s take an in-depth journey into what this method is, why it’s crucial for materials science and green chemistry, and how it opens doors for both global industries and local laboratories. Whether you’re a science enthusiast, researcher, educator, or business leader, the following guide contains reliable knowledge, clear steps, and actionable insights.
New Eco-Friendly Method Creates Giant Fullerenes Cheaply
| Feature | Detail / Statistic |
|---|---|
| Breakthrough Method | Eco-friendly electrochemical synthesis of giant fullerenes (up to 190 carbon atoms) at room temperature |
| Inputs Used | Readily available: graphite, ethanol, water, sodium hydroxide; no toxic solvents/catalysts |
| Energy Requirement | Ambient temperature (no high heat/furnaces needed) |
| Cost Impact | Significant cost reduction versus traditional high-temperature or solvent-based methods |
| Environmental Benefits | Lowered emissions and chemical waste; process is scalable and green |
| Professional Impact | Opens affordable production for R&D and industries: electronics, solar cells, nanomedicine |
| Access Official Info | FAPESP Official Release |
The eco-friendly electrochemical method for producing giant fullerenes is more than a technical marvel—it’s a turning point, making world-class nanotechnology accessible, affordable, and sustainable. Students, researchers, and companies can now explore fullerene innovations at a scale and price that aligns with both scientific ambition and environmental responsibility. As adoption grows, expect to see these green nanomaterials power future breakthroughs in medicine, electronics, energy, and beyond.
Understanding Fullerenes: Nature’s Carbon Wonders
Fullerenes are remarkable molecules composed solely of carbon atoms, joined in patterns that create hollow spheres, tubes, or ellipsoids. The best-known, C60 (dubbed the “buckyball”), resembles a tiny soccer ball made up of 60 carbon atoms. Discovered in 1985, fullerenes are found in everything from candle soot to cloud dust in interstellar space—demonstrating their inherent stability and versatility.
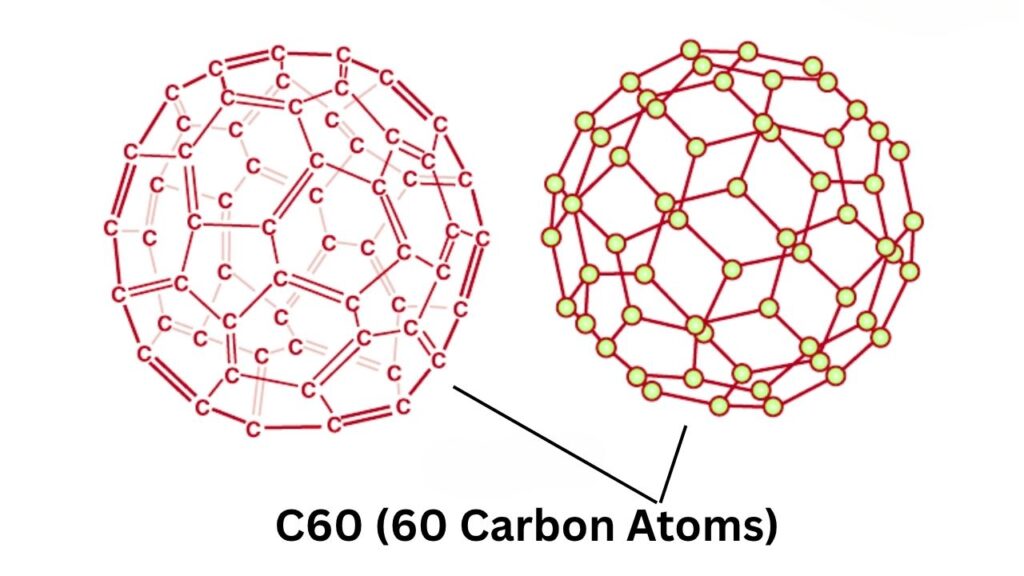
Why Are Fullerenes So Special?
- Exceptional Strength & Resilience: Their geometric structure allows them to withstand enormous pressures and absorb energy.
- Superb Electrical Properties: Fullerenes conduct electricity and can be modified for specific electronic behavior, making them ideal for organic solar cells, transistors, and flexible screens.
- Functional Versatility: Their hollow interiors and surfaces can be “decorated” with other atoms or molecules, opening horizons for targeted drug delivery, sensors, and advanced coatings.
For years, large fullerenes or “giant” fullerenes (with 70–190 carbon atoms or more) were highly sought after, but tough to synthesize in large quantities. Traditional synthesis consumed lots of energy, created hazardous waste, and required expensive equipment—putting these fascinating nanomaterials beyond the reach of many scientists and most applications.
Breaking Free from the Old: Drawbacks of Traditional Fullerene Production
Understanding what makes this new method revolutionary requires a quick look at the old ways:
1. Arc Discharge
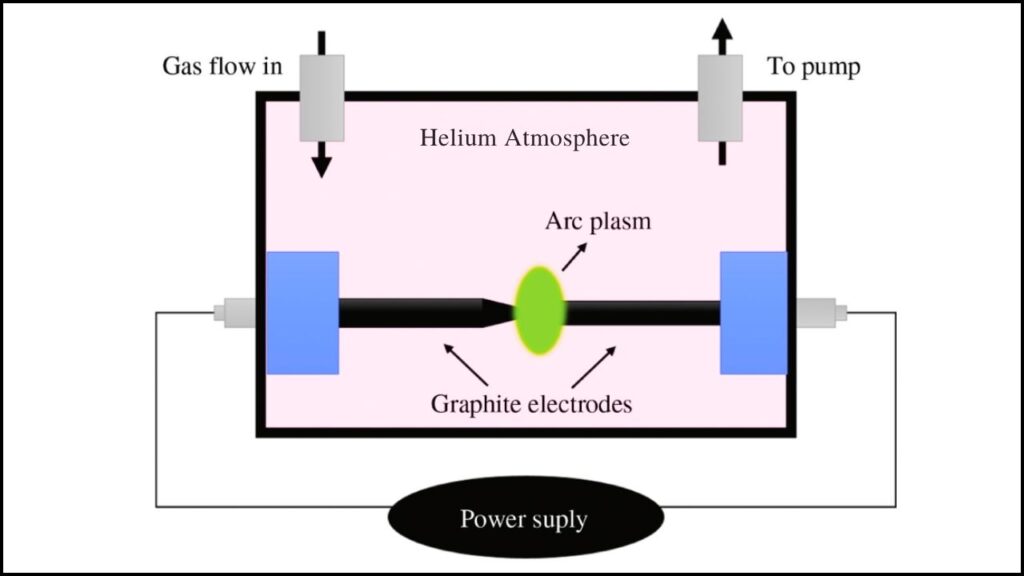
- Process: Two graphite rods, placed in a helium atmosphere, are zapped with massive electrical currents at >3,000°C. This produces a sooty residue, from which fullerenes are extracted.
- Drawbacks: Consumes a tremendous amount of energy, involves dangerous voltages, generates toxic chemical waste (often using benzene or toluene as solvents), and yields are inconsistent.
2. Laser Ablation
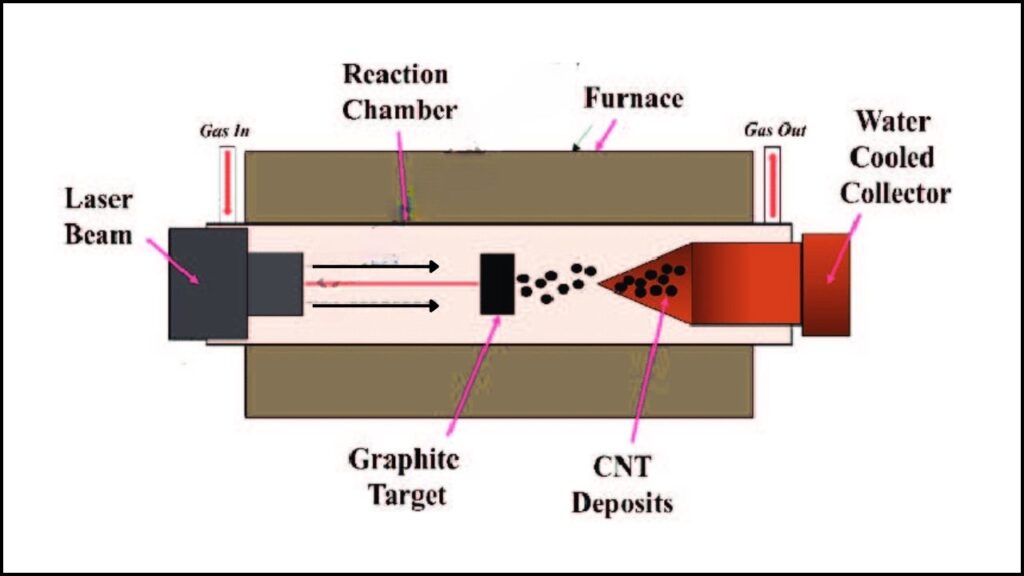
- Process: Pulsed lasers vaporize graphite targets at high pressure; carbon vapor cools to form fullerenes.
- Drawbacks: Needs costly, high-maintenance lasers, operates at extreme temperatures and pressure, and produces limited quantities.
3. Combustion or Chemical Vapor Deposition
- Process: Hydrocarbon gases (like acetylene) are burned in controlled ways; soot contains a mix of fullerenes and unwanted byproducts.
- Drawbacks: Process reliability and purity are low, and much chemical separation is needed.
In sum: All classic methods struggle with high costs, complex procedures, poor scalability, and significant environmental impact. Even obtaining single grams of large fullerenes could cost thousands of dollars and generate harmful waste.
The New Eco-Friendly Electrochemical Method: A Science and Sustainability Breakthrough
In 2025, researchers from the University of São Paulo (USP), Brazil, in collaboration with “La Sapienza” University of Rome, introduced a transformative synthesis method. This approach, published in Diamond and Related Materials and showcased by official innovation agencies, allows for the creation of giant fullerenes in an ordinary lab setting—no hazardous chemicals or scorching heat required.
The Process, Step by Step
1. Gathering Safe, Simple Ingredients
- Graphite: The same carbon material used in pencils.
- Ethanol: Common alcohol, both safe and widely available.
- Water: Clean, distilled water as solvent.
- Sodium Hydroxide: Also known as lye (used in soap-making and drain cleaners), handled with normal lab safety.
2. Building the Electrochemical Cell
- Mix ethanol, water, and sodium hydroxide in a glass vessel.
- Place the graphite (anode) and a suitable cathode into the solution.
3. Applying the Electric Current
- Mild, controlled electrical current is applied through the cell at room temperature—no heating or pressurization involved.
- The electrical process gently removes carbon atoms from the graphite, allowing them to reorganize and self-assemble into fullerenes in the solution.
4. Collecting and Purifying Fullerenes
- The fullerene-rich solution naturally separates from the graphite.
- Simple chemical or filtration procedures extract and purify the fullerenes, which often retain oxygen “handles”—useful for further modifications or attachments.
What Makes This Process Green?
- No toxic solvents, no rare chemicals, and no heavy metals.
- Operates at room temperature, reducing energy usage by up to 90% compared to arc discharge.
- Waste produced is minimal and largely non-hazardous.
- Scalable and reproducible: Any standard chemistry lab—or industrial facility—can adopt the technique with modest investment and straightforward training.
Real-World Example
A school laboratory could use this method to create fullerenes safely for teaching and experiments, while an industry-scale setup could produce kilograms for electronic manufacturing, all with a drastically reduced carbon footprint.
Why This Matters: Environmental and Professional Impact
For Scientists and Students
- Doubling Access: The high price and environmental impact of traditional fullerene synthesis meant many labs simply couldn’t afford or justify their use. The new method removes those barriers.
For Industry
- Unlocking the Market: Fields like flexible electronics, photodetectors, and nanomedicine have been limited by fullerene scarcity and cost. Lower-cost, greener fullerenes open the door to new products, rapid prototyping, and global competition.
For the Planet
- Climate and Waste Reduction: By eliminating harmful byproducts and slashing energy use, this new method aligns with international sustainability goals and green chemistry principles.
Expanded Applications: A World of Possibility
The ability to cheaply manufacture giant fullerenes transforms entire industries:
Electronics & Energy
- Solar Cells: Fullerenes act as electron acceptors; cheaper, purer fullerenes mean affordable and more efficient solar panels.
- Flexible Circuits: Used in organic thin-film transistors for foldable displays and wearable tech.
Medicine & Health
- Smart Drug Delivery: The hollow interior of fullerenes can encapsulate drugs, releasing them only at targeted cells—a future vision for cancer therapy.
- Bioimaging: Functionalized fullerenes serve as contrast agents, enabling clearer imaging of tissues and organs.
Environmental Tech
- Sensors: Fullerene-based devices detect pollution, toxins, and DNA mutations at extremely low levels, helping protect health and the environment.
- Advanced Composites: Added to polymers, fullerenes dramatically boost strength, flexibility, and chemical resistance without added toxicity.
Academic Research
- Nobel-winning studies on superconductivity, quantum computing, and nanorobotics all require access to high-purity nanocarbons like fullerenes. Affordable synthesis can catalyze new discoveries.
Professional, Laboratory & Industry Implementation Guide
- Plan Procurement: Source quality graphite, reagent-grade ethanol and sodium hydroxide, and distilled water.
- Assemble Setup: Use a beaker or glass cell with suitable electrodes (graphite for the anode), and a standard power supply.
- Prepare Solution: Mix ethanol, water, and sodium hydroxide as per published ratios (available in detailed scientific literature).
- Conduct Reaction: Apply a stable voltage (exact parameters available on request to suit scale/lab safety).
- Process and Purify: Extract fullerenes using filtration, precipitation, or chromatography—no hazardous solvents required.
- Analyze Quality: Characterize the product using spectroscopy or electron microscopy for purity and structure verification.
Safety Tip: Although ethanol, graphite, and sodium hydroxide are much safer than many traditional chemicals, always follow basic chemical safety procedures—wear gloves, goggles, and work in a ventilated area under teacher or technician supervision.
Comparison: Old vs. New in Fullerene Synthesis
| Feature | Traditional Methods | New Eco-Friendly Method |
|---|---|---|
| Temperature | >2000°C | Room temperature |
| Chemicals | Rare gases, strong acids/solvents | Ethanol, water, sodium hydroxide, graphite |
| Energy Use | Extremely high | Low |
| Process Complexity | High | Straightforward |
| Scalability | Difficult/expensive | Easy and modular |
| Byproducts | Lots of toxic and solid waste | Minimal, mostly water and safe carbon residue |
| Cost per gram | $500–$1,200+ | Estimated at <$50 (lab scale), much less in bulk |
| Environmental Footprint | Large | Drastically reduced |
| Customizability | Limited | High; oxygenated functional groups can be tailored |
| Global Impact | Restricted to elite labs | Accessible to schools, startups, and researchers |
Researchers Synthesize Elusive Cyclic Tri-Phosphirenium Ion, Advancing Main-Group Chemistry
New Polymer Breakthrough Boosts Durability and Sustainability Using Green Chemistry
Engineered Ruthenium Catalysts: A Sustainable Revolution for Hydrogen and Ammonia
FAQs About New Eco-Friendly Method Creates Giant Fullerenes Cheaply
What exactly is a fullerene, and what does “giant” refer to?
A fullerene is a carbon molecule arranged like a hollow cage (“buckyball” or sphere) or tube. “Giant” fullerenes are much larger versions with up to 190 or more carbon atoms, giving them unique properties for high-tech uses.
Does the new method compromise on quality or properties of fullerenes?
No. Studies show the structure, purity, and chemical tunability of fullerenes produced electrochemically match or exceed industry standards. The added oxygen groups can actually enhance certain applications.
Can this electrochemical fullerene synthesis method scale to industry?
Yes, easily. The method’s simplicity means factories can adopt it with little adaptation. Multiple cells can run in parallel, increasing production to kilograms or more per batch—unheard of with old methods at reasonable cost.
Is this method safe for school labs?
With standard lab precautions, yes. It’s far safer than previous fullerene synthesis, which involved hazardous solvents, open flames, or dangerous gases.