Messenger RNA, or mRNA, technology has transformed medicine by enabling vaccines and therapies that instruct our cells to make crucial proteins needed to fight diseases. Now, a groundbreaking AI tool named RiboNN has emerged, dramatically speeding up the development of mRNA-based treatments for viruses, cancers, and genetic disorders. Created through a partnership between The University of Texas at Austin and Sanofi, this innovative AI model allows scientists to predict how effectively different mRNA sequences produce proteins in specific cell types, offering unprecedented precision and efficiency in developing next-generation medicines.
This article breaks down what RiboNN is, how it works, and why it matters—not just for professionals but also for everyday readers curious about the future of healthcare. We explain complex science in simple terms, provide practical insights, and guide you through how AI is revolutionizing mRNA therapeutics, with clear examples and actionable knowledge for medical researchers, clinicians, and patients alike.
Table of Contents
New AI Tool Supercharges mRNA Treatments
| Topic | Details |
|---|---|
| New Tool Name | RiboNN |
| Developed By | The University of Texas at Austin & Sanofi |
| Purpose | Predict how efficiently mRNA sequences produce proteins in various cell types |
| Benefits | Faster mRNA therapeutic/vaccine design, higher protein yields, better targeting of specific organs |
| Scope of Application | Viruses (e.g., COVID-19), cancers, genetic disorders |
| Published In | Nature Biotechnology (2025) |
| Training Data Size | Over 10,000 experimental mRNA translation measurements |
| Future Potential | Personalized mRNA medicines, organ-specific therapies, streamlined clinical trials |
| Official Website for Reference | The University of Texas at Austin News |
The revolutionary AI tool RiboNN marks a major breakthrough in the field of mRNA therapeutics by accurately predicting protein production efficiencies across diverse cell types. This capability accelerates vaccine and drug design, making treatments for viruses, cancers, and genetic disorders faster, more personalized, and more effective. The collaboration between The University of Texas at Austin and Sanofi highlights how curiosity-driven science combined with AI can lead to life-changing healthcare innovations.
For researchers, clinicians, and patients, RiboNN offers new hope for precision medicine and paves the way toward a future where tailored mRNA therapies become standard care.
What is mRNA and Why Is It So Important in Medicine?
mRNA is a type of molecule that acts like a biological instruction manual. It tells cells which proteins to make, and proteins are the building blocks and workers involved in nearly every process inside our bodies. When scientists use mRNA in medicine, they create synthetic sequences that teach cells to produce proteins that can:
- Alert the immune system to fight off viruses or cancer cells.
- Replace faulty or missing proteins in people with genetic disorders caused by protein deficiencies.
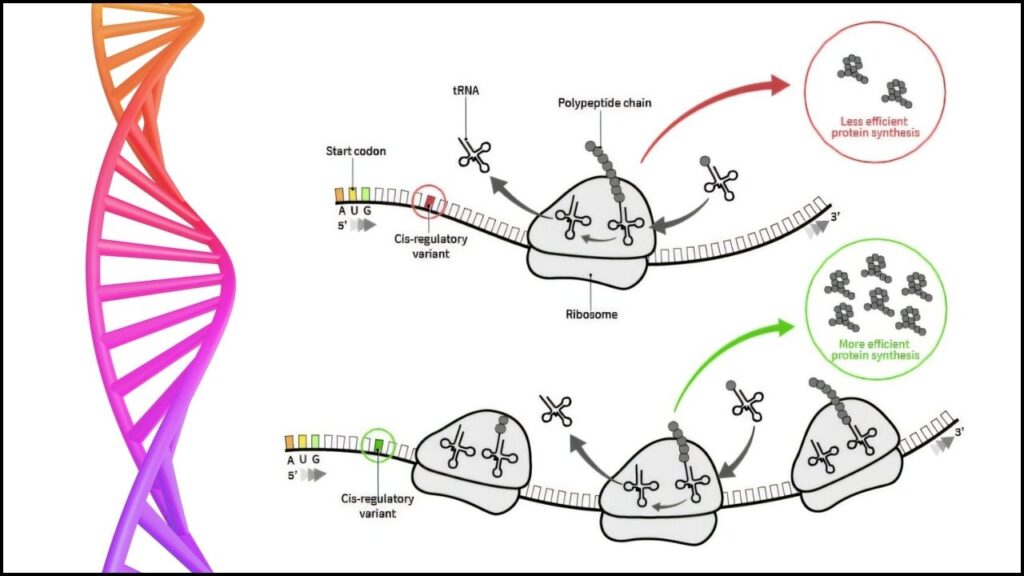
This technology is at the heart of many modern vaccines, including those for COVID-19. However, developing effective mRNA treatments involves complex challenges. Not all mRNA sequences produce proteins equally well in every type of cell, and targeting the right organs or tissues is essential for therapeutic success.
Introducing RiboNN: The AI That Predicts Protein Production
RiboNN is a sophisticated artificial intelligence (AI) model designed to predict how efficiently a given mRNA sequence will generate proteins in different cell types. Developed after analyzing more than 10,000 experimental results on mRNA translation efficiency across human and mouse cells, this tool helps scientists anticipate the protein output of mRNA designs before moving into costly laboratory testing.
How Does RiboNN Work?
- Data Collection: Researchers assembled a comprehensive dataset known as RiboBase, compiling thousands of verified experiments that measured how well various mRNA sequences produced proteins.
- Machine Learning Model: AI experts at UT Austin and Sanofi built a neural network that “learns” the complex patterns in this data, understanding which sequence features affect protein production.
- Prediction and Optimization: Scientists input potential mRNA sequences, and RiboNN estimates which ones will generate the highest protein levels in specific target cells or organs, such as the liver or heart.
- Design Refinement: Guided by RiboNN’s predictions, researchers can tweak and improve mRNA sequences computationally, dramatically reducing the need for trial-and-error experiments.
Dr. Can Cenik, co-leader of the project, emphasizes that this advancement was built on years of curiosity-driven research, providing a strong foundation for future breakthroughs.
Why RiboNN Changes the Game for mRNA Therapeutics
The introduction of RiboNN offers several critical advantages:
- Faster Development Timelines: Traditional mRNA design involves multiple cycles of laboratory testing and refinement, which can take months or years. RiboNN’s predictive ability cuts this time drastically by enabling in silico optimization.
- Precision Targeting: Diseases often affect specific organs, and RiboNN helps tailor mRNA sequences to maximize protein production in those particular tissues, increasing therapeutic efficacy and safety.
- Enhanced Protein Production: By identifying designs that yield higher protein levels, treatments become more potent, meaning lower doses are needed, reducing side effects and costs.
- Personalized Medicine: AI models like RiboNN open the door to mRNA therapies customized for individual patients, especially important in cancers where unique tumor proteins can be targeted.
- Streamlined Clinical Trials: Predictive modeling helps in selecting promising drug candidates and suitable clinical trial participants, increasing trial success rates and speeding regulatory approval.
Step-by-Step Guide: How Scientists Use AI for mRNA Therapy Design
To better understand how AI fits into mRNA treatment development, here is a simplified breakdown:
Step 1: Gather Extensive Experimental Data
Scientists collect comprehensive data on how various mRNA sequences perform in producing proteins across different cell types.
Step 2: Train the AI Model
Machine learning experts use this data to teach a neural network—like RiboNN—to recognize patterns of effective mRNA translation.
Step 3: Propose mRNA Candidates
Biotechnologists design initial mRNA sequences based on the protein needed to treat the disease.
Step 4: Predict Protein Output
RiboNN evaluates each sequence, estimating protein expression levels in targeted cells or organs.
Step 5: Optimize mRNA Designs
Using the AI’s feedback, researchers refine sequences to increase stability, potency, and organ targeting.
Step 6: Laboratory Testing
Optimized sequences move into lab experiments, including cell cultures and animal models, to validate AI predictions.
Step 7: Clinical Trials
Successful candidates proceed to human testing, where AI continues to support trial design and patient selection.
Real-World Examples of AI-Enhanced mRNA Therapies
- COVID-19 Vaccines: The rapid creation of effective mRNA vaccines for COVID-19 demonstrated the power of mRNA technology. Tools like RiboNN promise to make future vaccines even faster and more targeted in response to emerging viral strains.
- Cancer Immunotherapy: Personalized mRNA vaccines teach the immune system to recognize the proteins unique to a patient’s tumor. By optimizing mRNA sequences with AI, vaccine efficacy is enhanced.
- Genetic Disorders: In diseases caused by missing or defective proteins, AI-designed mRNA can instruct cells to efficiently produce the needed proteins, offering new therapeutic avenues.
Scientists Use DNA as a Building Block to Create Customized 3D Materials with Unique Properties
Genetically Engineered Plants Now Show Higher Efficiency for Sustainable Biofuel Production
FAQs About New AI Tool Supercharges mRNA Treatments
Q1: What makes mRNA different from traditional medicine?
A1: Unlike pills or antibodies, mRNA therapies guide your own cells to produce therapeutic proteins directly, offering flexible and precise treatment possibilities.
Q2: Why is AI important in mRNA development?
A2: AI helps analyze vast datasets and predicts which mRNA sequences will work best, speeding up development and improving accuracy beyond trial-and-error methods.
Q3: Can RiboNN predict protein production in all cell types?
A3: RiboNN was trained on datasets covering many cell types and can provide predictions for a wide range of tissues, aiding organ-specific treatment design.
Q4: How soon will personalized mRNA treatments be widely available?
A4: Personalized mRNA medicine is an active research area, with tools like RiboNN accelerating progress. Broad clinical availability depends on ongoing trials and regulatory approvals.
Q5: Does RiboNN replace laboratory experiments?
A5: No, it complements laboratory work by focusing efforts on the most promising mRNA sequences, saving time and resources.