Scientists have achieved a groundbreaking breakthrough by superheating gold to an incredible temperature of 33,740°F (19,000 Kelvin)—more than 14 times its normal melting point—without it melting. This stunning feat overturns a 40-year-old physics theory known as the entropy catastrophe limit and opens exciting new opportunities in physics, materials science, and other fields.

Introduction: What Happened with Gold?
Gold usually melts at about 1,947°F (1,337 K). For decades, physicists believed metals like gold could not be heated more than roughly three times their melting point without turning liquid. According to the entropy catastrophe theory, beyond that limit, the solid’s entropy (disorder) would exceed that of the liquid, forcing it to melt immediately.
In a new experiment, researchers rapidly heated an ultra-thin gold film using an ultra-fast laser pulse lasting only 45 femtoseconds (45 quadrillionths of a second), pushing its temperature to 33,740°F (19,000 K). Remarkably, the gold remained solid in that brief instant despite the extreme heat, challenging long-held scientific assumptions.
Gold Survives 33,740°F
| Feature | Details |
|---|---|
| Temperature achieved | 33,740°F (19,000 K) |
| Gold melting point | 1,947°F (1,337 K) |
| Superheating multiple | Over 14 times melting point |
| Laser pulse duration | 45 femtoseconds (45 quadrillionths of a second) |
| Sample thickness | ~50 nanometers |
| Experiment location | SLAC National Accelerator Laboratory, USA |
| Measurement technique | X-ray scattering of atomic vibrations |
| Scientific impact | Overturned 40-year-old entropy catastrophe theory |
| Applications | Fusion energy, planetary science, high-energy physics |
| Official source | Nature Journal |
The remarkable experiment superheating gold to over 33,740°F (19,000 K) without melting has shattered a long-standing 40-year-old physics theory. Thanks to ultra-fast laser heating and precise X-ray measurements, scientists revealed that gold’s melting limit depends on heating speed, not just temperature.
This finding advances fundamental physics and opens doors to new technologies in fields like fusion energy, planetary science, and material engineering. It highlights how bold experiments continue to expand our understanding of the natural world.
How Did Scientists Superheat Gold Beyond Limits?
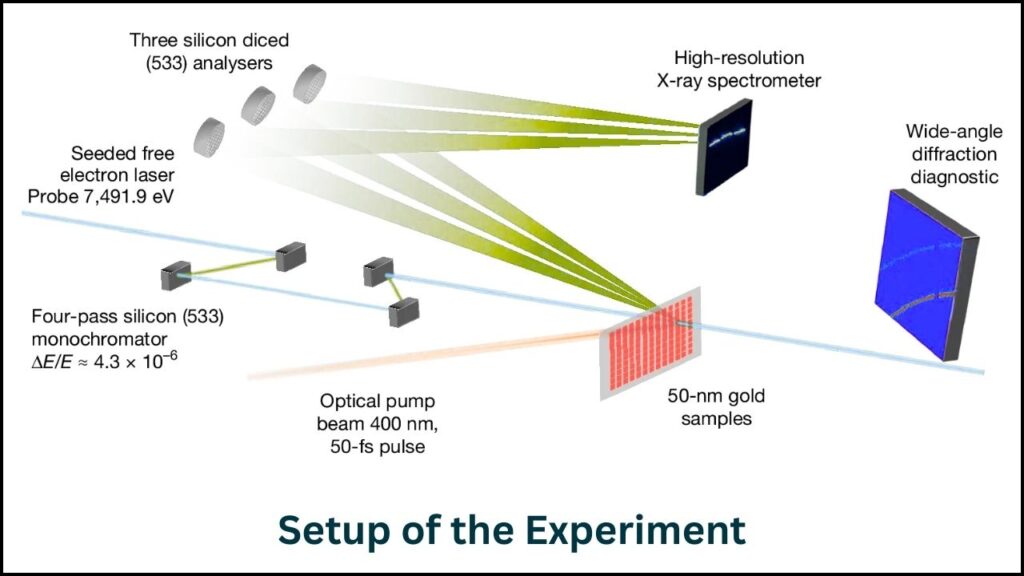
Step 1: Ultra-Fast Laser Heating
Scientists used an exceptionally powerful laser at the SLAC National Accelerator Laboratory to heat a gold foil only about 50 nanometers thick with a pulse lasting 45 femtoseconds. This heating was so rapid that the gold couldn’t physically expand, which normally would increase atomic disorder and cause melting.
By raising the temperature this fast, the gold’s atoms vibrated intensely but remained locked in their solid crystalline arrangement because there was no time for the structure to break down.
Step 2: Measuring Temperature via X-Ray Scattering
Right after the laser flash, the team fired ultrabright X-rays at the gold. By analyzing the scattering patterns of the X-rays off vibrating gold atoms, scientists precisely measured how fast the atoms were moving, revealing the exact temperature in the superheated state.
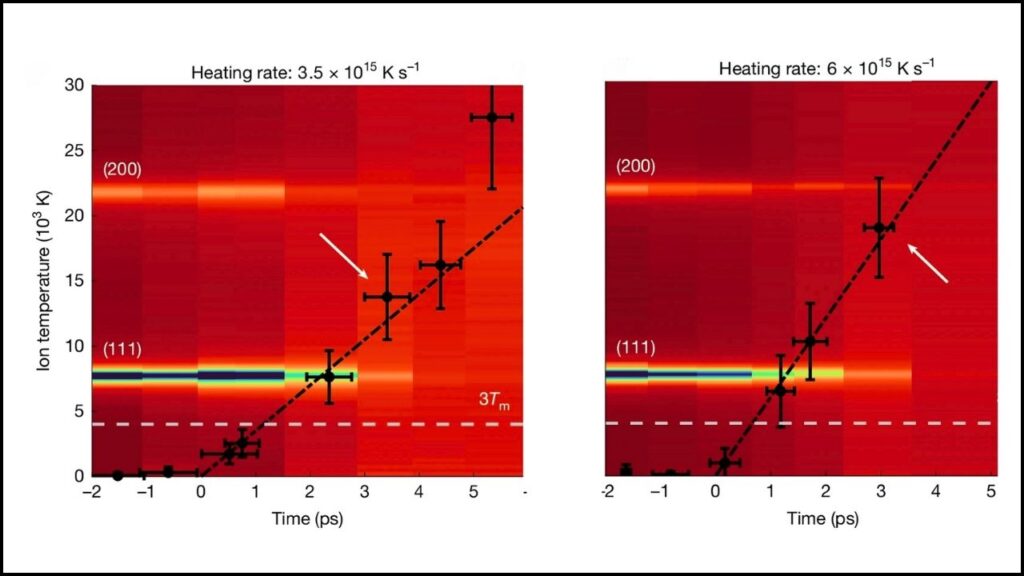
This marked the first time researchers could directly measure the temperature of such “warm dense matter,” a state seen in stars, fusion reactors, and planetary cores.
Why This Challenges Established Physics
Since the 1980s, the entropy catastrophe theory held that solids cannot be stably heated beyond about three times their melting point. This is because entropy—a measure of atomic disorder—should surpass that of the liquid phase, violating the second law of thermodynamics, which predicts spontaneous melting.
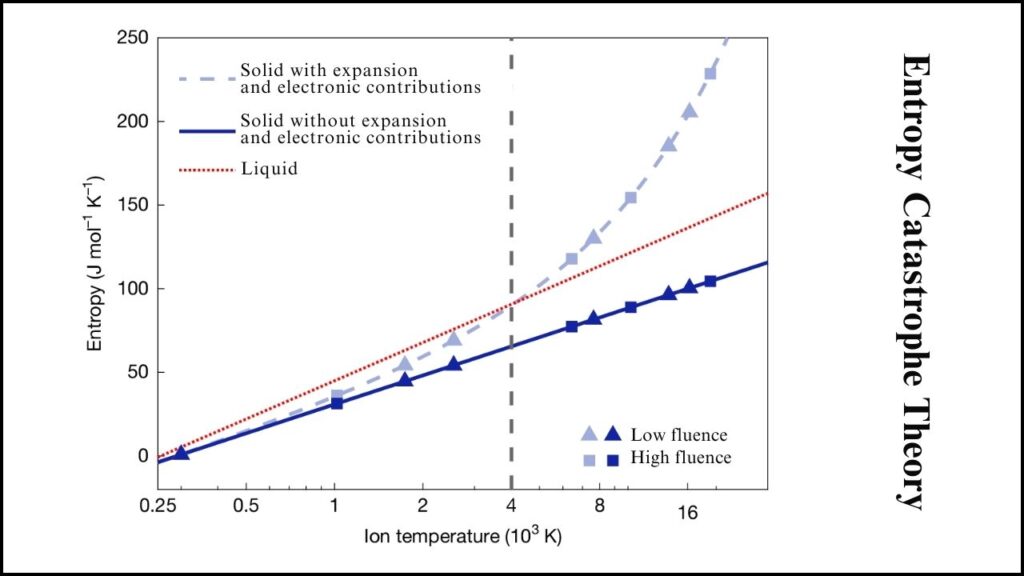
The recent experiment disproves this by demonstrating that gold can be superheated to over 14 times its melting temperature without melting when heated quickly enough. The gold existed momentarily in a unique non-equilibrium state that lies between solid and liquid.
This discovery forces a reconsideration of how matter behaves at extreme temperatures and pressures, rewriting decades of theoretical physics on the topic.
Practical Implications for Science and Technology
This discovery is more than theoretical; it has significant practical impacts:
- Fusion Energy Research: Understanding material behavior under extreme heat is crucial for designing fusion reactors, a potential clean energy source.
- High-Energy Physics: It helps model and simulate extreme states of matter found in stars and nuclear explosions.
- Planetary Science: The results aid in modeling conditions deep inside planets, where materials exist under intense heat and pressure.
- Materials Engineering: Insights from superheating may enable developing new materials and alloys with enhanced heat resistance or novel properties.
Understanding the Science: A Simple Guide
What Is Superheating?
Superheating is when a solid is heated above its normal melting point but does not turn to liquid. Usually, heating causes atoms to move apart and the structure to break down, leading to melting. However, if the heating is extremely rapid, atoms vibrate intensely but do not rearrange or expand, so the solid endures temporarily.
Why Does Heating Speed Matter?
- When materials are heated slowly, atoms rearrange into a liquid.
- If heated faster than atoms can move and rearrange, the material stays in a solid-like state for a short time.
This rapid heating prevents the expansion and subsequent rise in entropy that cause melting.
How Long Does This Superheated State Last?
The gold remained solid for just a trillionth of a second — enough to collect experimental data but too brief for practical applications currently. Theoretically, if expansion could be permanently inhibited, superheating could extend much further.
New Timed Laser Method Lets Scientists Hit ‘Pause’ on Ultra-Fast Silicon Melting
Magnetic Breakthrough: Scientists Watch Skyrmion Lattices Melt in Real Time
FAQs About Gold Survives 33,740°F
Q1: What temperature does gold normally melt at?
Gold melts at 1,947°F (1,337 K).
Q2: What is the entropy catastrophe theory?
It is a decades-old theory predicting solids cannot be heated beyond about three times their melting point without melting due to exceeding entropy limits.
Q3: How was the gold heated so rapidly?
Using an ultra-fast laser pulse lasting 45 femtoseconds, which delivered extreme energy almost instantaneously.
Q4: How was the temperature measured?
By firing ultrabright X-rays at the heated gold and measuring how atomic vibrations affected the X-ray scattering pattern.
Q5: Does this challenge the laws of thermodynamics?
No. The second law of thermodynamics remains intact; the experiment shows that rapid heating can temporarily prevent the expected entropy-driven melting.
Q6: What are the possible applications of this discovery?
It will help improve fusion research, planetary models, high-energy physics experiments, and the creation of heat-resistant materials.