A groundbreaking discovery has rocked the world of nuclear physics: a record-breaking “watermelon”-shaped atomic nucleus, known as astatine-188 (188At). This newly identified nucleus is the heaviest known atomic nucleus that emits a proton, shedding new light on the structure and behavior of matter at its most fundamental level. But what exactly is this watermelon nucleus, how was it discovered, and why does it matter? This article breaks down everything in an accessible yet authoritative way, making complex science easy to grasp for everyone—from curious 10-year-olds to seasoned professionals.

Table of Contents
What Is Astatine-188 and Why Is It Special?
Astatine-188 is an exotic isotope of the element astatine, consisting of 85 protons and 103 neutrons. The discovery is remarkable because astatine-188 is the heaviest nucleus to ever exhibit proton emission, a rare form of radioactive decay where a proton is ejected from the nucleus. This discovery provides new insights into nuclear stability and structure which could challenge or reshape existing atomic theory.
Here’s the exciting part: unlike most nuclei that are roughly spherical, 188At has a strongly prolate shape, meaning it looks like a stretched-out watermelon rather than a sphere. This unusual “watermelon” shape strongly influences how the nucleus behaves and how tightly it holds onto its particles. The shape deformation affects the binding energy—the energy that glues the nucleus together—leading to behaviors never observed before in heavy nuclei.
Record-Breaking “Watermelon” Nucleus Discovered
| Aspect | Details | Reference |
|---|---|---|
| Nucleus | Astatine-188 (188At) | University of Jyväskylä, Finland |
| Atomic Composition | 85 protons, 103 neutrons | Research published in Nature Communications |
| Shape | Strongly prolate (“watermelon-shaped”) | Theoretical nuclear physics models |
| Decay Type | Proton emission (rare radioactive decay type) | Measured via RITU recoil separator |
| Production Method | Fusion-evaporation reaction, silver target bombarded by strontium-84 (84Sr) ions | Finnish Accelerator Laboratory |
| Half-life | Approximately 190 microseconds | Detailed nuclear decay measurements |
| Scientific Impact | Advances understanding of nuclear structure and challenges current atomic models | International collaboration involving IIT Roorkee, University of Jyväskylä |
| Further Research | Plans to study heavier isotopes like astatine-189 | Theoretical and experimental follow-ups planned |
| Official Reference | University of Jyväskylä |
The discovery of the record-breaking watermelon-shaped atomic nucleus, astatine-188, signals an exciting era in nuclear physics. This heaviest known proton emitter challenges existing theories about nuclear shape and stability, revealing new realms of quantum interactions previously unobserved in heavy nuclei. Through international collaboration and state-of-the-art experimental techniques, scientists are redefining the limits of matter, providing fresh insights that could revolutionize atomic theory and its applications.
How Was This Discovery Made?
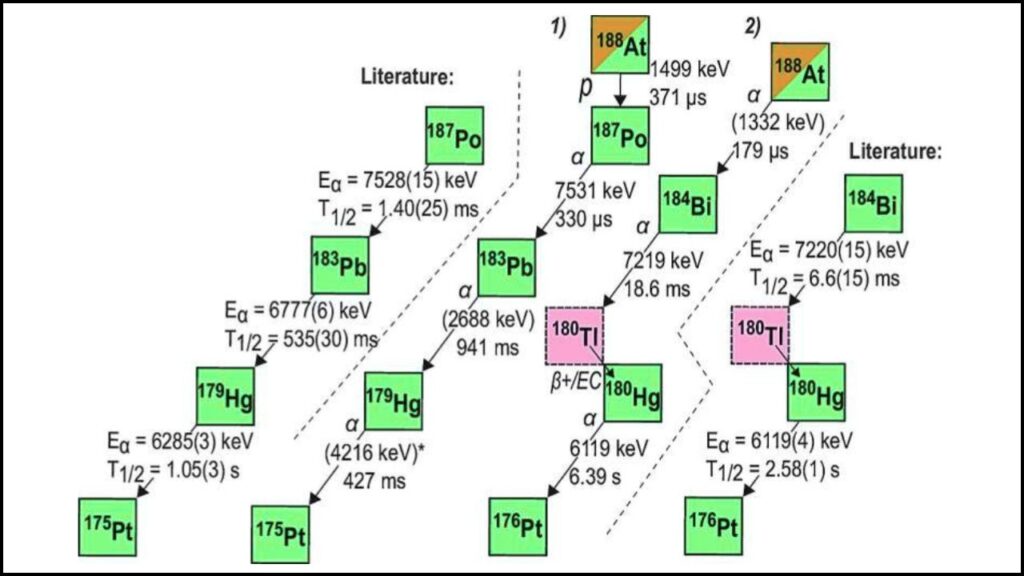
Creating such rare nuclei is no small feat. The team at the University of Jyväskylä in Finland used a fusion-evaporation process, where a natural silver target was bombarded with a high-energy beam of strontium-84 ions. During these high-speed collisions, the nuclei fused, and then “evaporated” three neutrons, forming the new isotope 188At.
Detecting this short-lived, rare isotope required highly sensitive equipment, specifically the RITU recoil separator, which isolates and identifies exotic nuclei almost immediately after formation. These experiments are notoriously difficult, relying on precision and sophisticated theoretical models to confirm the findings.
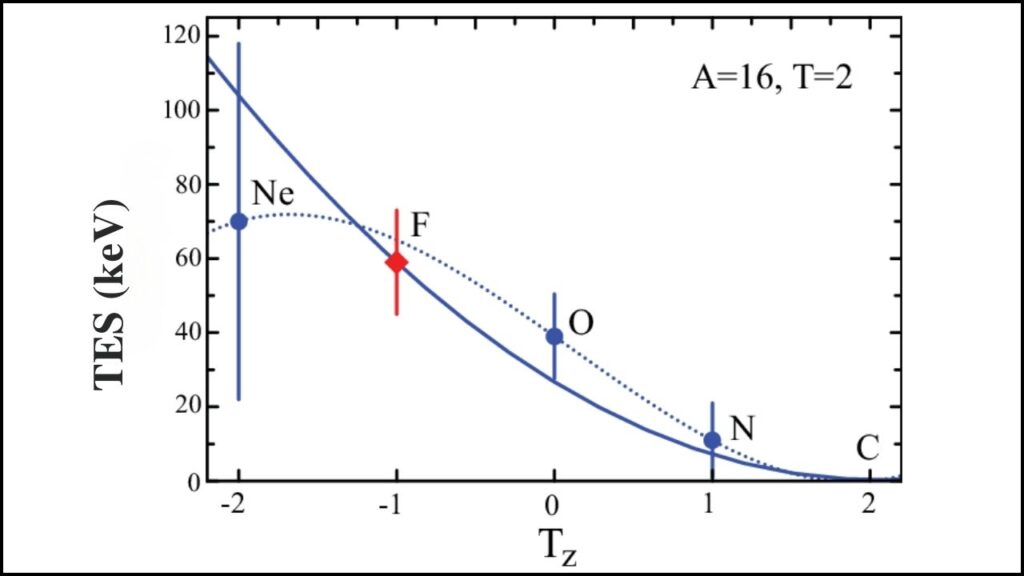
The team’s theoretical models indicated an unexpected quantum mechanical interaction, known as the Thomas-Ehrman shift, influencing the unusual proton binding energy in 188At. This quantum effect has not been observed before in such heavy nuclei, making the discovery doubly significant.
Why Does the Shape Matter?
Nuclei are typically imagined as spherical clusters of protons and neutrons, but in reality, many have deformed shapes. The degree and type of deformation can greatly affect a nucleus’s properties, including stability and decay modes.
The “watermelon” shape of 188At is a vivid illustration of a strongly prolate nucleus. This elongation means the nucleus is stretched along one axis, much like how a watermelon looks compared to a basketball.
Nuclear shapes affect energy levels and how tightly particles are held within the nucleus. The watermelon shape indicates that the binding energy of the outermost proton changes in a way that existing models did not predict, suggesting a new kind of interaction in these heavy nuclei. Understanding this helps scientists refine atomic models and theoretical physics, improving predictions about the behavior of matter under extreme conditions.
Breaking Down Proton Emission
Unlike alpha or beta decay, proton emission is a less common and more exotic form of radioactive decay. It occurs when a proton inside the nucleus has enough energy to escape, helping the nucleus move toward a more stable state.
Here’s a simple way to understand it:
- Imagine a crowded room (the nucleus) filled with protons and neutrons.
- Sometimes, the room becomes so crowded that some protons are pushed out (emission) to balance the space.
- This ejected proton takes energy away, changing the mass and structure of the nucleus.
Because proton emission is so rare, observing it in such a heavy isotope like 188At provides unique data for nuclear theory.
What Does This Mean for Atomic Theory?
This discovery opens the door for potentially rewriting parts of atomic theory, especially regarding the form and limits of nuclear stability. Traditional models assumed more uniform shapes and predictable energy trends for nuclei. The watermelon nucleus defies some of those assumptions.
Scientists now have new evidence showing that nuclei can be more diverse in shape and behavior than previously known, especially as they become more proton-rich. The research also validates and extends important quantum mechanical effects like the Thomas-Ehrman shift, which affects how nuclear particles interact near stability limits.
Future studies aim to observe even heavier isotopes like astatine-189, which might also emit protons, further pushing the boundaries of nuclear physics. These insights will not only deepen our fundamental understanding but also improve applications in nuclear medicine, energy, and materials science.
Practical Implications and Career Insights for Aspiring Nuclear Physicists
This discovery demonstrates the importance of international collaboration, meticulous experimental procedures, and theoretical modeling in nuclear physics research. For students and professionals interested in careers in this field:
- Get strong training in both experimental and theoretical physics.
- Learn to use or develop sophisticated equipment like recoil separators and particle detectors.
- Engage in computational modeling to predict nuclear behaviors.
- Pursue opportunities in accelerator laboratories or universities specializing in nuclear research.
Institutions like the University of Jyväskylä and IIT Roorkee offer excellent programs and partnerships in this niche yet vital research area.
MIT Just Proved Einstein Was Wrong — Quantum Physics Shattered Again
New Quantum Weirdness Spotted in Superconductor—Could It Upend Physics?
Scientists Freeze Quantum Motion Without Cooling — A Physics First
FAQs About Record-Breaking “Watermelon” Nucleus Discovered
What is proton emission and how common is it?
Proton emission is a rare radioactive decay where a proton is ejected from an unstable nucleus to achieve greater stability. It occurs mostly in proton-rich nuclei and is much less common than alpha or beta decay.
Why is the watermelon shape important?
The watermelon shape (strongly prolate deformation) represents a highly elongated nucleus that alters how nuclear forces bind protons and neutrons, affecting decay and stability in ways not accounted for by simpler spherical models.
How was the watermelon nucleus created?
Scientists bombarded a silver target with high-energy strontium ions. The fusion of these produced 188At, which was quickly detected using specialized equipment called the RITU recoil separator.
Can this discovery affect medical or industrial applications?
While the discovery is fundamental physics, understanding exotic nuclei helps improve nuclear technologies applied in medicine (like cancer treatment) and energy production.
What future research is planned?
Researchers plan to investigate heavier isotopes like astatine-189 and further study the decay processes and shapes of heavy nuclei through experiments and theoretical models.