Ammonia production is a cornerstone of modern industry, essential for fertilizer manufacturing and a potential green energy carrier. Traditionally, ammonia synthesis requires high temperatures and pressures, consuming enormous energy and releasing significant carbon emissions through the Haber-Bosch process. However, recent advances with cobalt catalysts have paved the way for ammonia production under ambient conditions, offering a promising route for sustainable, energy-efficient, and environmentally friendly synthesis.
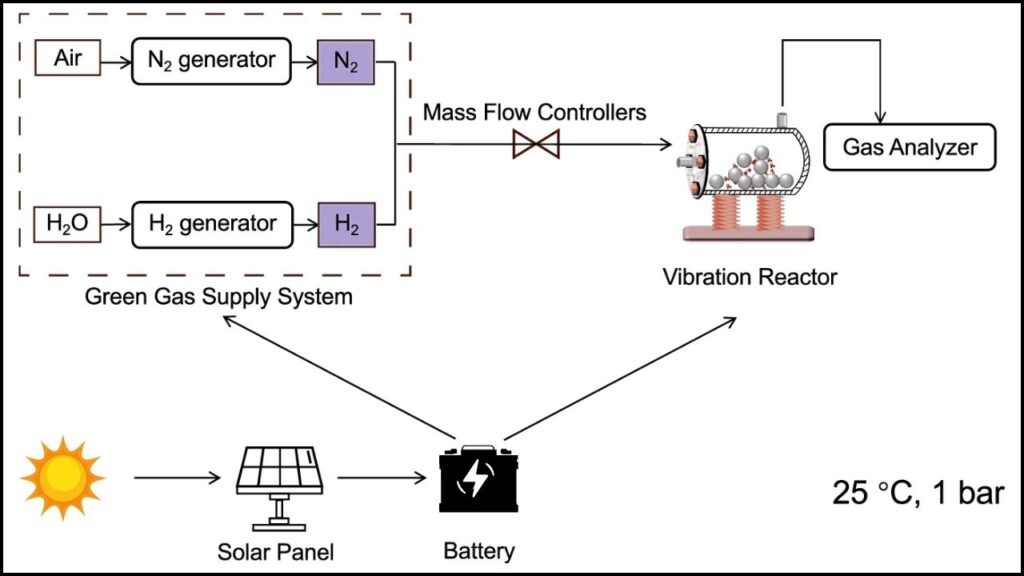
This article explores the groundbreaking role of cobalt catalysts in enabling ammonia synthesis at room temperature and atmospheric pressure. This revolutionary progress not only has the potential to transform chemical manufacturing but also supports global efforts to reduce carbon emissions and promote green energy.
Table of Contents
Cobalt Catalyst Enables Ammonia Production Under Ambient Conditions
| Feature | Details |
|---|---|
| Catalyst | Cobalt single clusters in nitrogen-doped carbon |
| Ammonia Yield Rate | 76.2 μg h⁻¹ mg⁻¹ |
| Faraday Efficiency | 52.9% under ambient conditions |
| Temperature & Pressure | Room temperature (25°C), atmospheric pressure (1 bar) |
| Mechanism | Electron back-donation from cobalt weakens N≡N bond, changing rate-determining step |
| Sustainability Impact | Enables energy-efficient, carbon emission-reducing ammonia synthesis |
| Official Catalyst Info | See detailed catalyst design and studies at Nature Communications |
The discovery of cobalt catalysts that enable ammonia production under ambient conditions marks a significant breakthrough in sustainable chemistry. By cleverly altering the reaction pathway and lowering energy barriers, cobalt-based catalysts offer a greener, energy-efficient alternative to the century-old Haber-Bosch process. This technology holds immense promise to reshape fertilizer manufacturing, support renewable energy storage, and contribute to global carbon reduction goals.
As research progresses towards commercial applications, cobalt catalysts stand at the forefront of innovative solutions addressing climate change and resource sustainability.
Why Is Ammonia Important?
Ammonia (NH₃) is widely used as a fertilizer, playing a vital role in global food production by enhancing soil nutrients. It also holds promise as a green energy carrier and fuel due to its high hydrogen content. However, current industrial methods to synthesize ammonia depend heavily on the Haber-Bosch process, which operates at temperatures around 400–500°C and pressures up to 200 atmospheres, using fossil-fuel-derived hydrogen. This process consumes approximately 1-2% of the world’s annual energy supply and accounts for substantial CO₂ emissions.
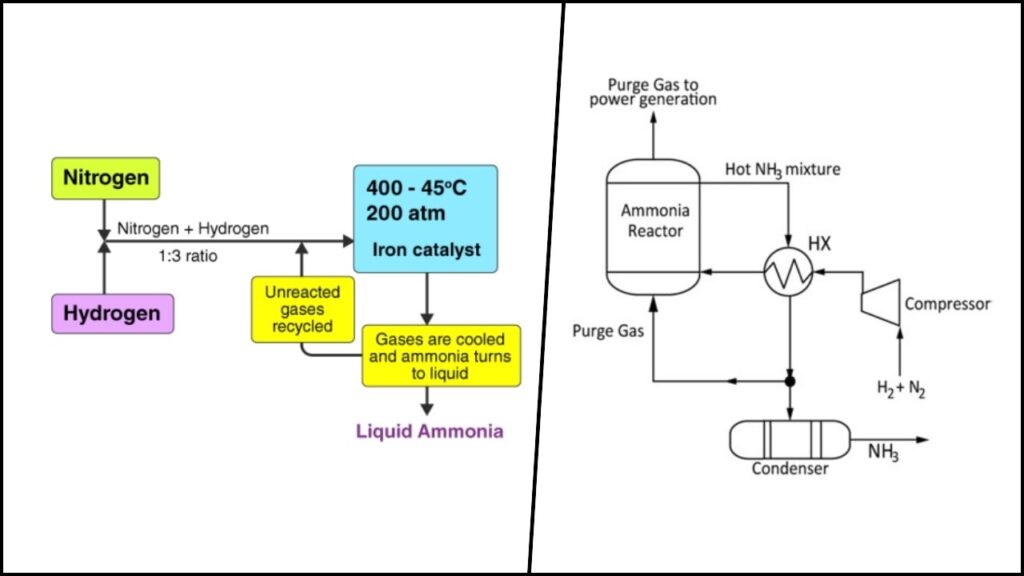
Therefore, developing efficient ammonia synthesis under ambient conditions is a significant scientific and environmental challenge. It promises to drastically reduce the energy required and cut greenhouse gas emissions associated with fertilizer production and beyond.
How Does Cobalt Catalyst Enable Ammonia Production Under Ambient Conditions?
Understanding the Challenge
The foremost barrier to ammonia synthesis from nitrogen gas (N₂) is breaking the very strong triple bond (N≡N) in nitrogen molecules. This bond requires a large amount of energy to dissociate, which is why traditional ammonia synthesis relies on extreme conditions.

Cobalt’s Role in Overcoming This Barrier
New research showcases how cobalt single-atom or single-cluster catalysts embedded in specially designed nitrogen-doped carbon frameworks act as powerful electron donors. These cobalt centers back-donate electrons into the antibonding orbitals of nitrogen molecules, weakening the triple bond and making it easier for nitrogen to split and react with hydrogen.
By altering the rate-determining step — the slowest and energy-intensive step in the nitrogen reduction reaction — cobalt changes the reaction kinetics. Instead of N₂ bond cleavage being the bottleneck, it shifts the challenge to subsequent proton addition, which is energetically more favorable under mild conditions.
Key Data and Performance
- Ammonia yield rate reaches an impressive 76.2 μg per hour per milligram of catalyst.
- Faraday efficiency, which measures how effectively electrical energy converts into chemical energy in ammonia, reaches 52.9% at room temperature and 1 bar pressure.
- The catalyst maintains stability and activity over multiple reaction cycles without noticeable degradation.
- Computational chemistry methods confirm the exothermic nature of nitrogen dissociation on cobalt single clusters.
Mechanism Visualization
- Cobalt atoms act as Lewis acid sites, attracting nitrogen molecules.
- Nitrogen approaches cobalt clusters with negligible energy barriers.
- Electron transfer weakens nitrogen bonds.
- Hydrogenation proceeds with lowered activation energy, producing ammonia efficiently.
Practical Implications for Industry and Environmental Sustainability
Advantages of Cobalt Catalysts for Ammonia Synthesis
- Reduced Energy Requirements: Enables ammonia synthesis at room temperature, drastically cutting energy use.
- Lower Carbon Emissions: Mitigates dependency on fossil fuels for hydrogen generation and extreme processing conditions.
- Economic Potential: Possibly lowers capital and operational costs compared to Haber-Bosch plants.
- Scalability: Compatibility with electrochemical systems allows integration with renewable electricity sources.
Steps for Implementing Ambient Ammonia Synthesis
- Catalyst Development: Manufacture cobalt single-cluster catalysts supported on nitrogen-doped carbon with high surface area.
- Electrochemical Setup: Assemble reactor systems that allow efficient nitrogen access and hydrogen delivery at ambient pressure.
- Reaction Optimization: Control applied potentials and reaction environments to maximize ammonia yield and minimize competing processes like hydrogen evolution.
- Scale-up and Integration: Build prototype reactors powered by renewable energy aiming for carbon-neutral fertilizer production.
New Carbon Catalyst Could Revolutionize Biodiesel — No Metals Needed
Frontier Supercomputer Runs 5 Million Simulations to Unlock Stronger, Lighter Carbon Fiber
FAQs About Cobalt Catalyst Enables Ammonia Production Under Ambient Conditions
What is the main benefit of using cobalt catalysts in ammonia production?
The main advantage is enabling ammonia synthesis under ambient conditions—room temperature and pressure—greatly reducing energy consumption and environmental impact.
How much ammonia can these cobalt catalysts produce?
Research shows a yield rate of about 76.2 micrograms per hour per milligram of catalyst at room temperature, which is competitive considering the mild conditions compared to industrial standards.
Is this technology ready for commercial use?
While highly promising, ambient ammonia synthesis with cobalt catalysts is still in experimental stages. Further work on scaling, durability, and reactor design is underway.
Can this method reduce CO2 emissions?
Yes. By eliminating the need for extreme heat and pressure, it reduces reliance on fossil fuels, thereby cutting greenhouse gas emissions significantly.