Methanol steam reforming has long been recognized as one of the most promising ways to produce clean hydrogen—a crucial energy source for the future. Recently, a cadmium single atom catalyst (SAC) has emerged as a groundbreaking advancement, dramatically improving the efficiency and stability of this process. This article will explore this exciting development, explain methanol steam reforming in an easy-to-understand way, and provide valuable insights for professionals and curious readers alike.
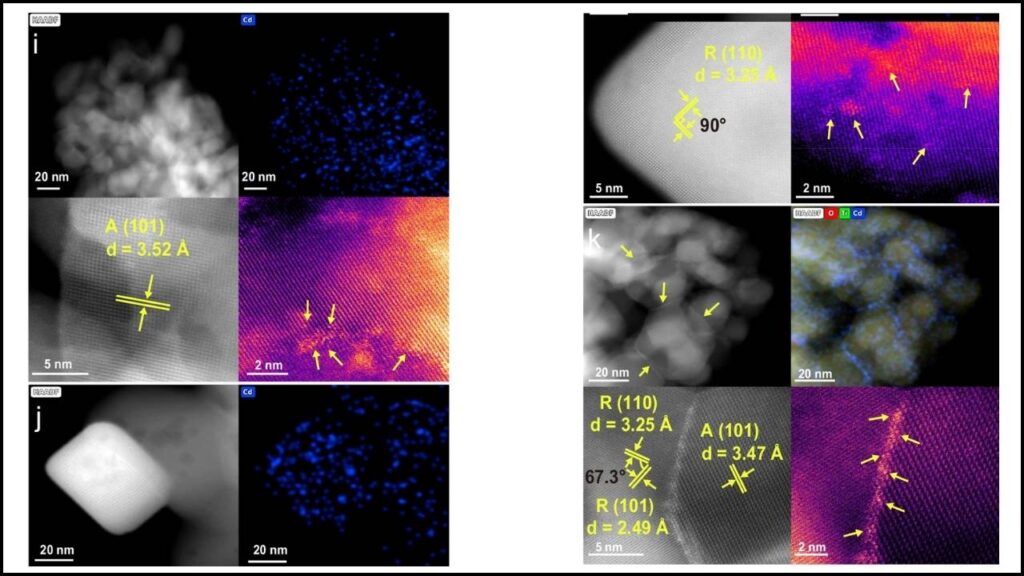
Table of Contents
What is Methanol Steam Reforming?
Methanol steam reforming (MSR) is a chemical process where methanol (CH3OH) reacts with water vapor (steam) to create hydrogen gas (H2) and carbon dioxide (CO2). The reaction requires heat and a catalyst to speed it up. The resulting hydrogen is a clean fuel that, when used, emits only water, making it desirable for energy transition and reducing pollution.
The overall simplified reaction is:CH3OH+H2O→3 H2+CO2CH3OH+H2O→3H2+CO2
Catalysts play a vital role in speeding up this reaction efficiently and with the lowest possible production of unwanted byproducts such as carbon monoxide (CO), which can poison fuel cells.
Cadmium Single Atom Catalyst Boosts Methanol Steam Reforming Efficiency
| Feature | Description | Data/Stats |
|---|---|---|
| Catalyst Type | Cadmium Single Atom Catalyst (Cd SAC) | Boosts hydrogen production rates by 8-15x compared to conventional catalysts |
| Methanol Conversion | Achieves 100% conversion rate | Extremely low CO output (~0.1 mol%) for clean hydrogen |
| Stability | Continuous operation for over 150 hours without loss of activity | Promising for industrial applications |
| Production Scale | Lab-scale catalyst scalable by 3D printing to kilogram scale | Enables potential commercial hydrogen production applications |
| Process Temperature | Steam reforming generally operates at 250-360 °C, 20 bar pressure | Requires external heat to drive endothermic reactions |
The discovery and application of cadmium single atom catalysts in methanol steam reforming mark a significant advance in clean hydrogen production technology. With superior conversion rates, minimal carbon monoxide output, excellent stability, and scalable manufacturing potential, this innovation offers a promising path for industries aiming for efficient and sustainable hydrogen generation. Embracing such cutting-edge catalysts can help accelerate the global transition toward a cleaner energy future.
Understanding the Cadmium Single Atom Catalyst Innovation
What is a Single Atom Catalyst?
Traditional catalysts involve clusters or particles of metals. Single atom catalysts (SACs) are a cutting-edge technology where individual metal atoms are dispersed on a support material. This design maximizes atomic efficiency—every atom is active—and can create unique catalytic properties due to altered electronic structures.
Why Cadmium?
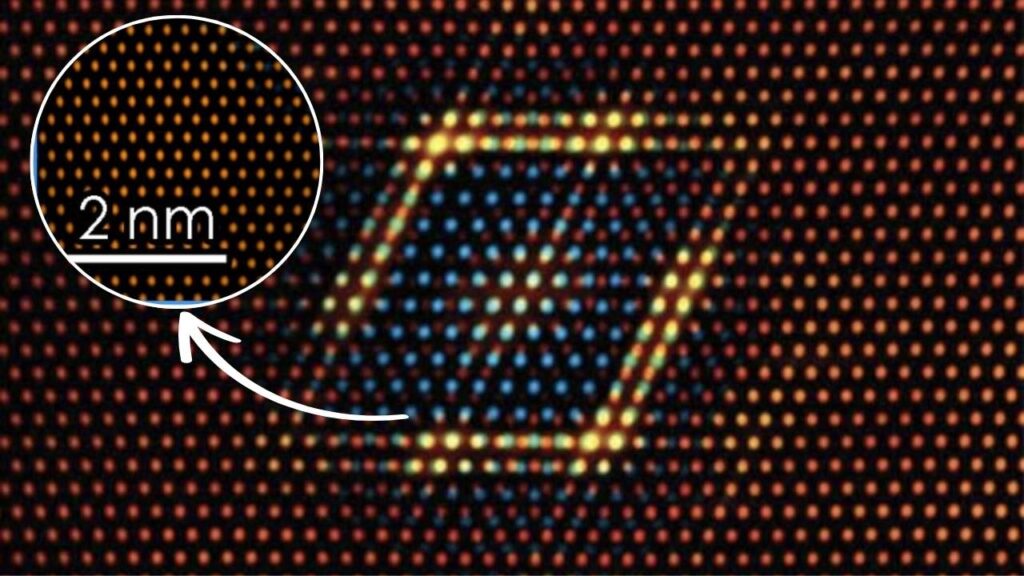
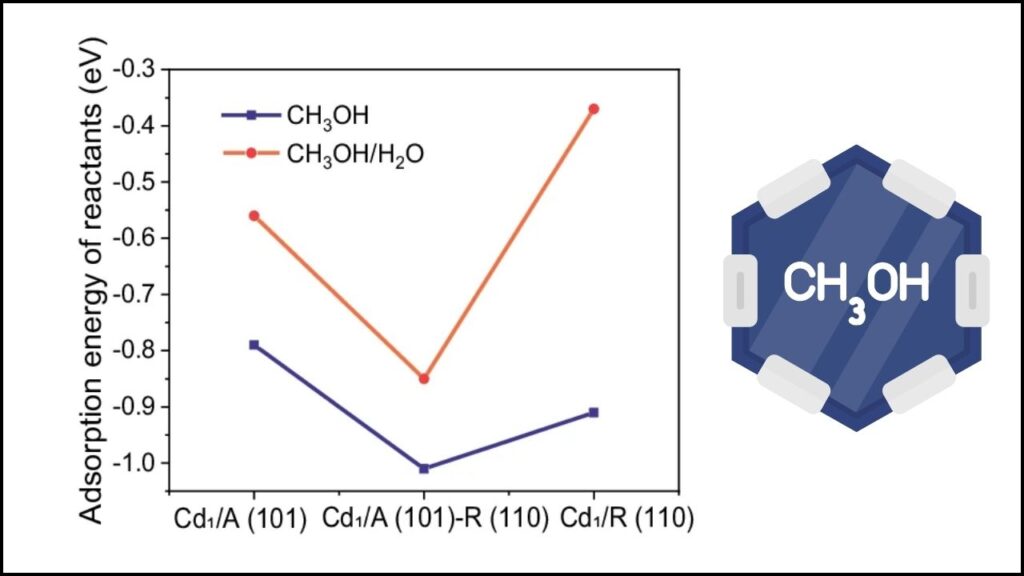
The recent breakthrough involves anchoring single cadmium atoms at the interface between different crystal facets of titanium dioxide (TiO2)—specifically anatase (101) and rutile (110) facets. This unique “phase interface” creates special Cd-O-Ti active sites with distinctive geometry and electronic features that dramatically enhance methanol steam reforming efficiency.
How Does Cadmium SAC Improve the Process?
- Boosted Methanol Conversion: Achieves 100% methanol conversion, ensuring no waste of feedstock.
- Higher Hydrogen Production: Produces hydrogen at rates up to 15 times higher than conventional anatase surface sites and 8 times higher than rutile surface sites.
- Lower Carbon Monoxide: Generates CO concentrations as low as 0.1 mol%, beneficial for downstream fuel cell use.
- Long-Term Stability: Runs continuously for over 150 hours without degradation, proving practical for industrial use.
- Interface Density: Pretreatment techniques increase interface density, further enhancing hydrogen output by 11%.
- Industrial Scalability: These catalysts can be 3D printed into kilogram-scale monoliths, allowing for practical hydrogen production use.
Step-by-Step Guide to Methanol Steam Reforming with Cadmium SAC
1. Feed Preparation
- A mixture of methanol and water (steam) is prepared, typically with a molar ratio of about 1:1 to 1.5:1.
- This mixture is vaporized and pressurized (~20 bar) for the reaction.
2. Catalytic Reaction
- The vaporized methanol-steam mixture enters a reactor containing the cadmium SAC supported on TiO2.
- The reaction occurs at temperatures between 250–360 °C.
- The cadmium single atoms at phase interfaces efficiently catalyze the breakdown of methanol and water into hydrogen and carbon dioxide.
3. Hydrogen Separation
- Hydrogen gas is separated from the product stream using pressure swing adsorption (PSA) or selective membranes.
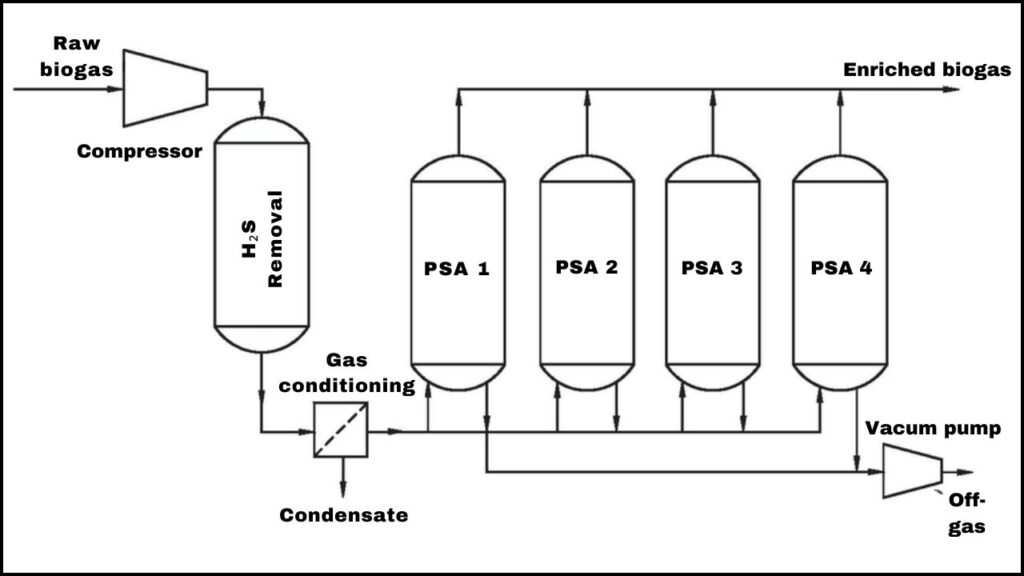
- The process yields pure hydrogen suitable for fuel cells or other applications.
4. Byproduct Management
- Carbon dioxide formed is typically captured or managed depending on the setup.
- Carbon monoxide production is minimized due to the efficiency of the Cd SAC catalyst, which is crucial for preventing fuel cell poisoning.
5. Continuous Operation and Scaling
- The catalyst demonstrated a long operational lifespan without losing efficiency.
- The ability to print the catalyst structure at large scales offers industrial feasibility.
Practical Advice for Industry Professionals
- Consider Interface Engineering: Enhancing catalyst performance by designing phase interfaces, such as those between anatase and rutile TiO2, can unlock significant improvements.
- Invest in Single Atom Catalysts: SACs provide high atom efficiency and unique active sites unachievable by larger metal particles.
- Balance Reaction Conditions: Maintain optimal temperature (250-360 °C) and pressure (~20 bar) for efficient steam reforming.
- Low CO Production is Vital: Use catalysts like Cd SAC to minimize carbon monoxide, essential for fuel cell quality hydrogen.
- Plan for Scalability: Use emerging manufacturing technologies like 3D printing for catalyst deployment at industrial scale.
Why This Matters: The Future of Clean Energy
Hydrogen is poised to be a cornerstone of a sustainable energy future, powering vehicles, industry, and homes with zero carbon emissions. Efficient and stable hydrogen production methods like methanol steam reforming, especially those boosted by innovations like cadmium single atom catalysts, will accelerate this transition. This breakthrough offers a practical, scalable solution to produce clean hydrogen on-demand with minimal environmental impact.
New MOF Catalyst Developed for Efficient C–O Cross Coupling Reactions
New Low-Cost Catalyst Discovered for Clean Hydrogen Energy: A Game-Changer for the Future
FAQs About Cadmium Single Atom Catalyst Boosts Methanol Steam Reforming Efficiency
What is Methanol Steam Reforming?
Methanol steam reforming is a process where methanol reacts with steam at high temperatures with a catalyst to produce hydrogen and carbon dioxide.
Why is Hydrogen Important?
Hydrogen is a clean fuel that emits only water when used, making it a key material for reducing pollution and fighting climate change.
What Makes Cadmium Single Atom Catalysts Special?
They involve single cadmium atoms anchored on a surface, creating highly active sites that improve efficiency, yield, and stability in producing hydrogen from methanol.
How Does This Benefit Industry?
It enables higher hydrogen production with fewer impurities, reduces costs by using less catalyst material, and provides long-term catalyst durability.
Can This Technology Be Scaled?
Yes, the catalyst can be manufactured through 3D printing into large-scale structures, suitable for commercial hydrogen production.