Artificial photosynthesis has long been hailed as a game-changing technology for clean energy. Now, scientists at the University of Basel in Switzerland have designed a groundbreaking molecule that can store four charges simultaneously under light—two positive and two negative. This advance promises to supercharge our ability to convert sunlight into carbon-neutral solar fuels like hydrogen, methanol, or synthetic gasoline. The discovery represents a vital step toward mimicking nature’s photosynthesis process more efficiently and practically than ever before.
In this article, we’ll explore the science behind this breakthrough molecule, why it matters, and how it can lead to a sustainable energy future. The article is structured clearly for easy understanding by all readers—whether a curious 10-year-old or an energy sector professional—while packing in detailed insights and practical examples.
Table of Contents
Breakthrough Molecule With Four Charges Could Supercharge Artificial Photosynthesis
| Topic | Details |
|---|---|
| Breakthrough | Molecule storing 4 charges (2 positive, 2 negative) simultaneously under light |
| Molecular Design | Five linked segments: electron donors, acceptors, and central light-absorbing chromophore |
| Charge Storage | Stepwise double light excitation with stable charge retention in near-solar intensity |
| Applications | Carbon-neutral solar fuels: hydrogen, methanol, synthetic gasoline |
| Challenges | Integration into full artificial photosynthesis systems still ongoing |
| Official Research Publication | Published in Nature Chemistry (DOI: 10.1038/s41557-025-01912-x) |
The remarkable creation of a molecule capable of simultaneously storing four charges under near-natural sunlight is a pivotal step forward in artificial photosynthesis. This breakthrough brings the dream of sustainable, carbon-neutral solar fuel production closer to reality by mimicking how plants elegantly convert sunlight into energy.
Such advances inspire hope for a cleaner, more sustainable energy future—one where sunlight powers our homes, cars, and industries without warming the planet. Though challenges remain, the foundation has been laid for exciting progress in renewable energy technology that could transform the global energy landscape in the coming decades.
What Is Artificial Photosynthesis?
Photosynthesis is the process by which green plants convert sunlight, water, and carbon dioxide into energy-rich sugars and oxygen. This natural energy conversion sustains nearly all life on Earth by powering food chains and recycling carbon dioxide.
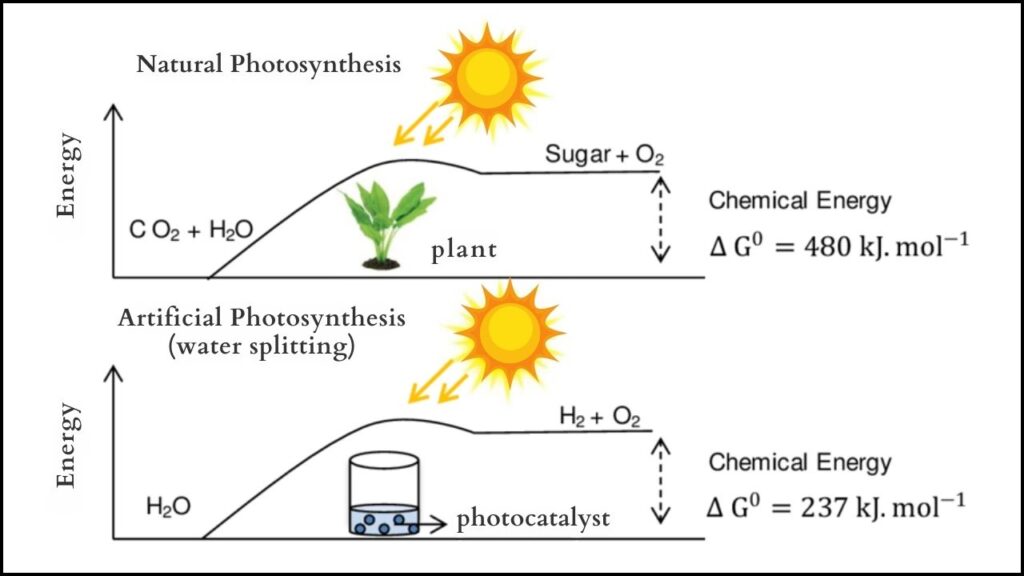
Artificial photosynthesis aims to recreate this process using technology. Instead of plants making sugar, the goal is to convert sunlight into carbon-neutral fuels—clean energy carriers like hydrogen that do not add net CO₂ to the atmosphere. Such fuels could power vehicles, industries, and homes sustainably, reducing reliance on fossil fuels that drive climate change.
Why Is This Breakthrough Molecule Important?
One of the biggest hurdles in artificial photosynthesis is managing multiple electric charges simultaneously to drive chemical reactions like splitting water into hydrogen and oxygen. Natural photosynthesis is highly efficient because it can accumulate and transfer several charges at once in a controlled way.
Until now, scientists struggled to design molecules that could hold more than two charges under sunlight levels that are practical for real-world use. The University of Basel team has overcome this by creating a molecule that stores two positive and two negative charges simultaneously with stable retention, even under dim, near-natural sunlight intensity.
This ability to hold multiple charges is crucial because:
- It increases energy storage capacity, enabling more powerful chemical reactions.
- Charge stability means the molecule’s energy can be harnessed for longer, improving efficiency.
- Operating under ordinary sunlight brings artificial photosynthesis closer to practical applications beyond lab settings.
How Does the Molecule Work?
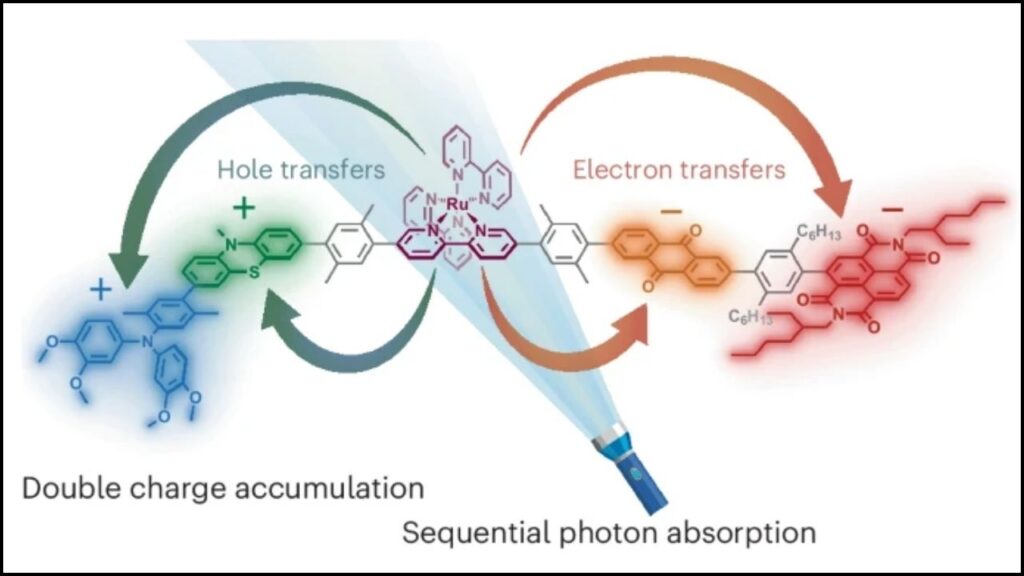
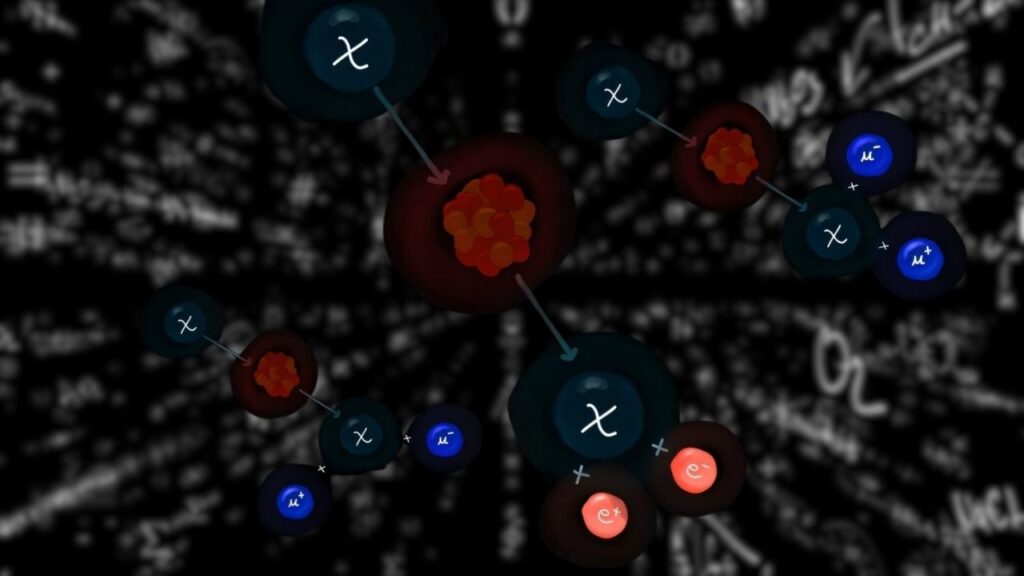
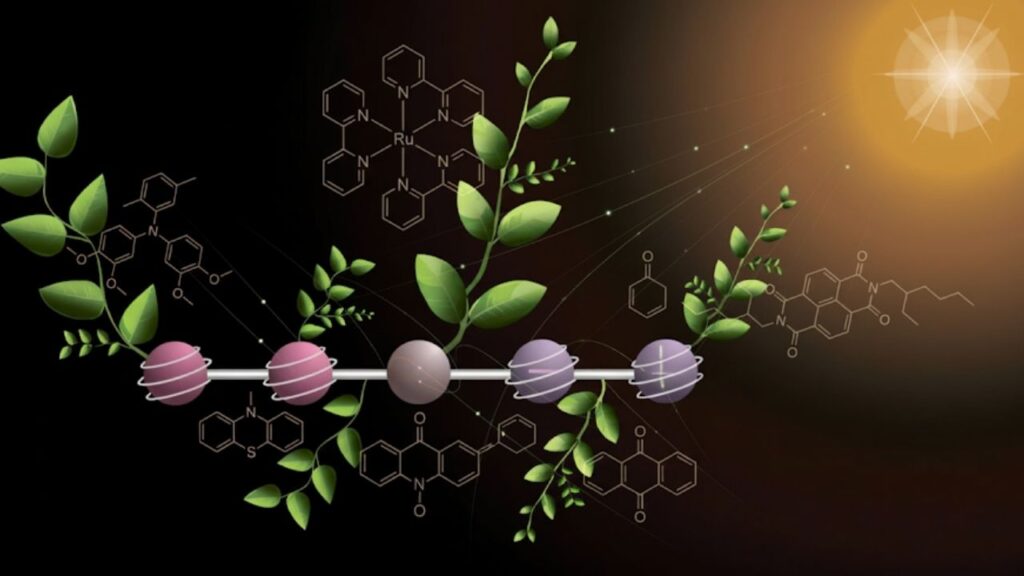
The molecule’s structure is a marvel of molecular engineering. It consists of five interconnected sections, each with a specialized function:
- Two Electron Donor Units — These release electrons and become positively charged.
- Two Electron Acceptor Units — These gain electrons and become negatively charged.
- Central Chromophore — A light-absorbing unit that captures sunlight and initiates the electron transfer process.
The process happens stepwise with two flashes of light:
- The first flash creates one positive and one negative charge, sending them to opposite ends of the molecule.
- The second flash repeats the reaction, resulting in two positive and two negative charges stored simultaneously.
This stepwise excitation allows the molecule to work efficiently even under weak lighting conditions similar to natural sunlight. Previous systems needed intense laser light, which is impractical for large-scale use.
Practical Implications for Clean Energy
With this molecule’s design, the stored charges can now be used to trigger important chemical reactions, such as:
- Splitting water molecules to produce pure hydrogen fuel.
- Converting carbon dioxide into sustainable fuels such as methanol or synthetic gasoline.
These fuels can be burned with zero net carbon emissions since the CO₂ released equals the CO₂ originally absorbed during fuel creation. This closes the carbon cycle and supports climate goals.
Challenges and the Road Ahead
While this molecule is a significant breakthrough, it is just one piece of the artificial photosynthesis puzzle. There remain challenges ahead:
- Integrating this molecule into fully functioning devices.
- Optimizing catalytic systems to turn stored charges into usable fuels efficiently.
- Scaling up from laboratory experiments to industrial applications.
However, the research offers critical insights into how electrons are transported and stored—knowledge essential for building effective solar fuel technologies.
Step-by-Step Guide to Understanding This Breakthrough
Step 1: Understanding Natural Photosynthesis
Plants use sunlight to power a series of chemical reactions turning water and carbon dioxide into sugar and oxygen. This involves moving positive and negative charges inside cellular reaction centers.
Step 2: Recreating Charge Storage in Technology
Scientists want to imitate these charge-storing reactions in molecules that can be used in devices, enabling sunlight-to-fuel conversion.
Step 3: Designing the Breakthrough Molecule
By linking 5 molecular parts for light absorption, electron release, and electron acceptance, researchers showcased stable four-charge storage—an impressive feat compared to previous two-charge systems.
Step 4: Optimizing for Real-World Conditions
The molecule works under dim light intensities close to natural sunlight, overcoming a major challenge for prior artificial photosynthesis models requiring lasers.
Step 5: Directing Charges to Create Fuel
Once charges are stored, they can power reactions like water splitting or CO₂ conversion into fuels, paving the way for carbon-neutral energy production.
Scientists Capture Quantum ‘Eternal Dance’ of Molecules for the First Time Ever
Fluorescent Molecules Withstand Extreme Environments Using New Technique
Bigger Molecules Now Proven to Extend Quantum Charge Flow Like Never Before
FAQs About Breakthrough Molecule With Four Charges Could Supercharge Artificial Photosynthesis
Q1: What is artificial photosynthesis, and why is it important?
Artificial photosynthesis mimics the natural photosynthesis process by converting sunlight, water, and CO₂ into useful fuels. It offers a sustainable alternative to fossil fuels, reducing greenhouse gas emissions.
Q2: What makes this new molecule special?
It can store four charges simultaneously under sunlight-like conditions—two positive and two negative—enabling more powerful chemical reactions essential for creating solar fuels.
Q3: How soon will artificial photosynthesis be available commercially?
While this discovery is a major step, further work is needed to integrate molecules into devices and scale production. Commercial availability may still be several years away.
Q4: What fuels can artificial photosynthesis produce?
Mainly carbon-neutral hydrogen, methanol, and synthetic gasoline, all of which emit no net CO₂ when used.
Q5: How does charge storage improve solar energy efficiency?
Storing multiple charges stabilizes energy long enough to drive complex fuel-producing reactions, increasing overall conversion efficiency.