Artificial Intelligence (AI) is transforming chemistry with a groundbreaking model called FlowER. Unlike traditional AI that tries to guess only the end products of chemical reactions, FlowER predicts every step of the reaction process by tracking how electrons move. This breakthrough makes exploring complex chemical reactions clearer, faster, and more accurate—an exciting development for creating new medicines, materials, and technologies.
Table of Contents
What Is FlowER and Why Does It Matter?
FlowER is an AI designed to understand chemical reactions by following the flow of electrons, which are the tiny particles responsible for chemical changes. Traditional AI approaches predict final products based on inputs, but sometimes these predictions don’t follow basic chemical laws like conservation of mass, where atoms cannot just appear or disappear.
Imagine reading a story by only knowing the first and last pages—that’s how older models worked. FlowER, however, reads the whole book, understanding each event along the way by mimicking the arrow-pushing technique chemists use when analyzing reactions. This method lets FlowER predict not just the main products but also the side products and impurities, which are very important in industries such as pharmaceuticals because impurities can affect drug safety.

The AI can also quickly learn new types of reactions with very few examples, similar to how humans learn – speeding up innovation and discovery in chemistry.
AI Powered Model
| Feature | Description | Data/Stats | Professional Relevance |
|---|---|---|---|
| AI Model Name | FlowER | Developed by Kookmin University & MIT | Critical for researchers and pharma R&D |
| Unique Capability | Predicts detailed electron movement in chemical pathways | Trained on ~1 million reaction cases | Ensures accuracy and mechanistic understanding |
| Learning Efficiency | Learns new reaction types with just 32 examples | >65% accuracy on unseen reactions | Accelerates chemical synthesis innovation |
| Applications | Predicts impurities and suggests condition adjustments | Adopted by pharmaceutical leaders | Improves drug safety and manufacturing quality |
| Open Science Approach | Tools and data shared openly on GitHub for research collaboration | Expansion planned for metal catalysts | Broadens global research cooperation |
FlowER represents a monumental leap in how chemistry is studied and applied. By mimicking human chemists’ understanding of electron flow, it brings unprecedented accuracy and insight into predicting chemical reactions, from lab research to industrial production. This technology promises faster innovation cycles, safer pharmaceuticals, greener materials, and more efficient chemical processes worldwide. Open-source collaboration ensures continued progress, making AI a key partner in the future of chemistry.
For official detailed insights, see the FlowER research published in Nature: Nature 2025, DOI: 10.1038/s41586-025-09426-9
How Does FlowER Work? A Step-by-Step Guide
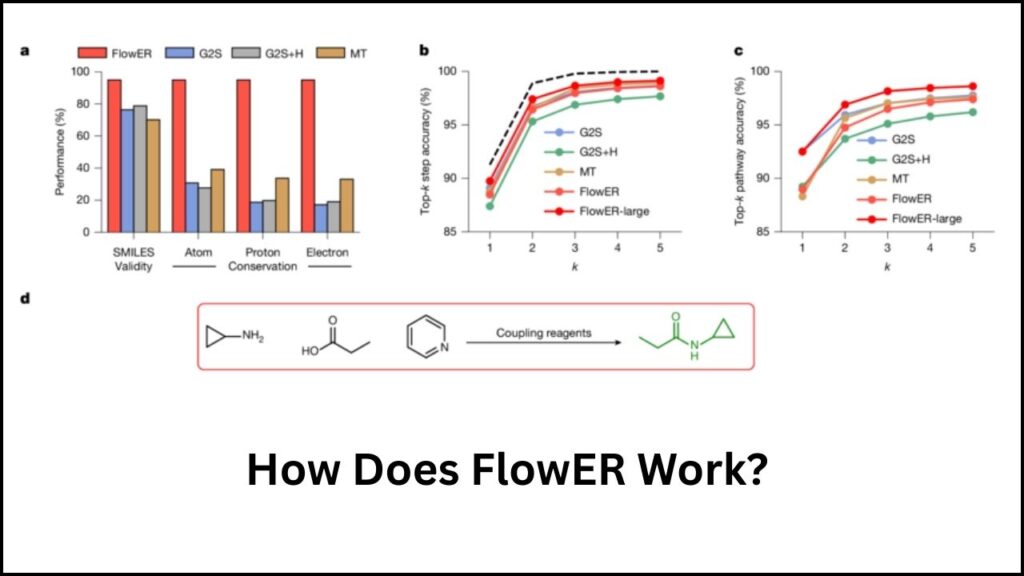
Step 1: Training with Real Chemical Reactions
FlowER was trained on about one million known chemical reactions. This rich dataset helped it learn the fundamental electron shifts that drive reactions, rather than just memorizing starting and ending materials.
Step 2: Electron Movement Tracking
FlowER uses a chemist’s method called “arrow pushing” to track electron flow, ensuring atomic and mass balance throughout every reaction step. This means no atoms disappear or appear mysteriously, respecting the essential laws of chemistry.
Step 3: Pathway Prediction
Instead of predicting just the final product, FlowER maps out every intermediate step and possible branch the reaction could take. This allows chemists to foresee byproducts and unwanted impurities early on, which saves time and resources in refining reactions.
Step 4: Learning New Reactions Quickly
This AI model can learn new types of reactions after seeing as few as 32 examples, showcasing an efficient and human-like learning ability. This accelerates the discovery of new synthetic pathways without needing large datasets.
Step 5: Industrial Application
Pharmaceutical companies use FlowER to predict potential impurities and optimize reaction conditions accordingly. By reducing impurities, FlowER helps ensure medications are safer and manufacturing processes more cost-effective.
Practical Advice: Using FlowER in Chemistry and Industry
- For Drug Developers: Predict impurities early to save time and reduce costly failures in drug formulation and testing.
- For Material Scientists: Explore novel synthetic routes for greener batteries and other advanced materials with confidence.
- For Educators: Teach students electron flow in reactions with a visual, AI-powered tool that mimics expert reasoning.
- For Researchers: Collaborate and build upon open FlowER tools available on GitHub to push the boundaries of AI-guided chemistry.
Beyond FlowER: Autonomous Exploration of Chemical Reaction Networks
AI also powers software like Chemoton, which uses quantum mechanics combined with AI to explore highly complex reaction networks automatically. These algorithms balance between exploring possible new pathways (breadth-first) and deepening knowledge on promising areas (depth-first), allowing chemists to uncover new catalysts and reaction conditions faster and more efficiently.
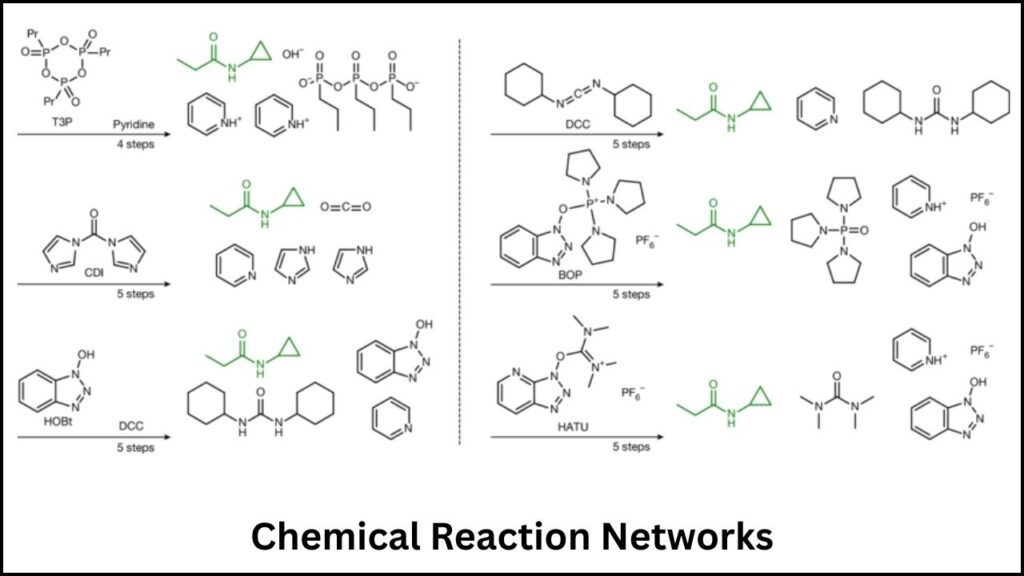
Such automation represents a significant step toward reducing the massive human and computational effort traditionally required to analyze chemical reaction mechanisms in detail.
Open-Source AI Models Are Secretly Hogging Way More Computing Power—The Truth Will Shock You
New QCBench Test Reveals Which AI Models Truly Excel at Step-by-Step Reasoning
Amazon Deploys Its 1 Millionth Robot and Launches New Generative AI Model for Smarter Warehousing
FAQs About AI Powered Model
Q1: How is FlowER different from other AI models in chemistry?
It tracks electron movement step-by-step, respecting chemistry laws while predicting entire pathways, not just end products.
Q2: In what ways does FlowER improve drug development?
It helps predict and reduce impurities that affect safety, thus streamlining drug testing and manufacturing.
Q3: How quickly can FlowER learn new types of chemical reactions?
With as few as 32 examples, FlowER can accurately predict previously unseen reactions.
Q4: Is FlowER available for researchers worldwide?
Yes, its developers have made tools publicly available on platforms like GitHub for global collaboration.
Q5: Which industries are benefiting most from AI-driven reaction exploration?
Pharmaceuticals, materials science, and chemical manufacturing sectors are seeing significant advances.