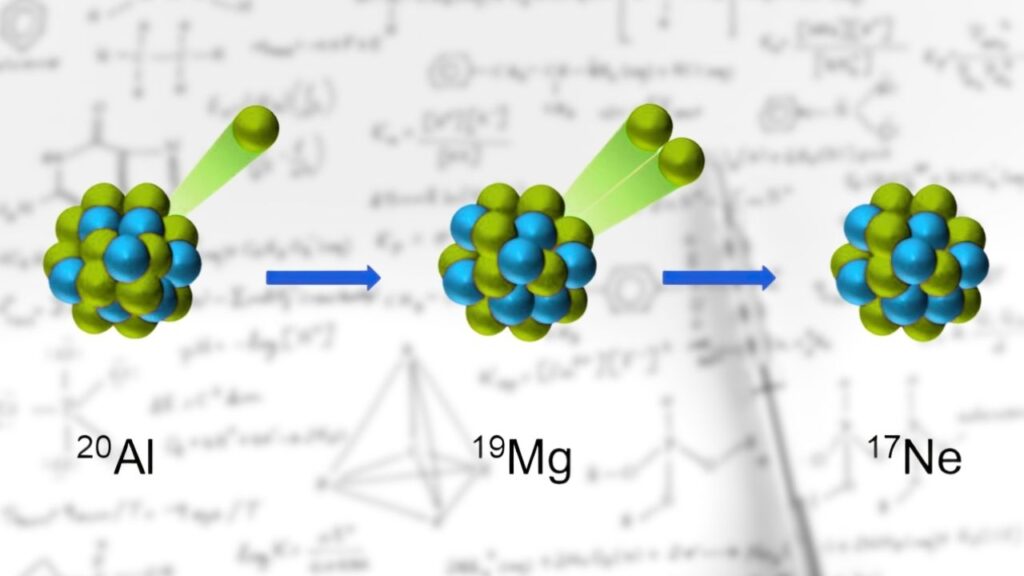
Aluminum is everywhere—in cans, kitchen foil, electronics, and even spacecraft. But scientists have just found something brand new: aluminum-20. This isn’t just a slightly different version of everyday aluminum. It’s the lightest, most unstable aluminum isotope ever detected, and it breaks down in a way no other nucleus ever has—by emitting three protons in a rare, cascading decay.
This discovery matters because it shows what can happen when atoms are pushed to their absolute limits, helping us understand not just the elements we know, but the ones that exist for only the tiniest sliver of time. It’s a reminder that the universe still has plenty of mysteries left for scientists to explore.
Table of Contents
Aluminum-20
| Feature | Details |
|---|---|
| Isotope Name | Aluminum-20 (²⁰Al) |
| Discovery Date | July 10, 2025 |
| Experiment Location | GSI Helmholtzzentrum für Schwerionenforschung, Darmstadt, Germany |
| Discovery Team | Xu et al., Institute of Modern Physics, Chinese Academy of Sciences |
| Decay Mode | Three-proton emission (cascade of one, then two protons) |
| Significance | First known case where the first decay product is also a two-proton emitter |
| Stability Status | Extremely unstable, far beyond the proton drip line |
| Scientific Impact | Reveals isospin symmetry breaking, challenges nuclear models |
| Relevance for Careers | Opens new research paths in nuclear physics, astrophysics, and technology |
| Official Reference | Physical Review Letters, July 10, 2025 (official article title: “Isospin Symmetry Breaking Disclosed in the Decay of Three-Proton Emitter ²⁰Al”) |
The discovery of aluminum-20 is a reminder that science is never finished. Every new experiment, every new isotope found, adds a piece to the puzzle of how our universe works. For students, it’s a story of curiosity and discovery. For professionals, it’s a chance to test and improve our understanding of matter itself.
What Are Isotopes, and Why Is Aluminum-20 Different?
Atoms are built from three main parts: protons, neutrons, and electrons. The number of protons defines the element, while the number of neutrons can vary, creating isotopes. Stable isotopes last a long time; unstable ones decay quickly.
Aluminum has several isotopes, but aluminum-20 is special. With only 13 protons and 7 neutrons, it’s much lighter than stable aluminum-27, which has 14 neutrons. This difference makes aluminum-20 extremely unstable. In fact, it’s so unstable that it cannot exist in nature—only under special laboratory conditions for a tiny fraction of a second.
How Was Aluminum-20 Discovered?
The discovery took place at the GSI Helmholtz Center for Heavy Ion Research in Darmstadt, Germany. Scientists accelerated a beam of atomic nuclei to nearly the speed of light and smashed them into a target. Among the particles produced, they detected a new isotope: aluminum-20.
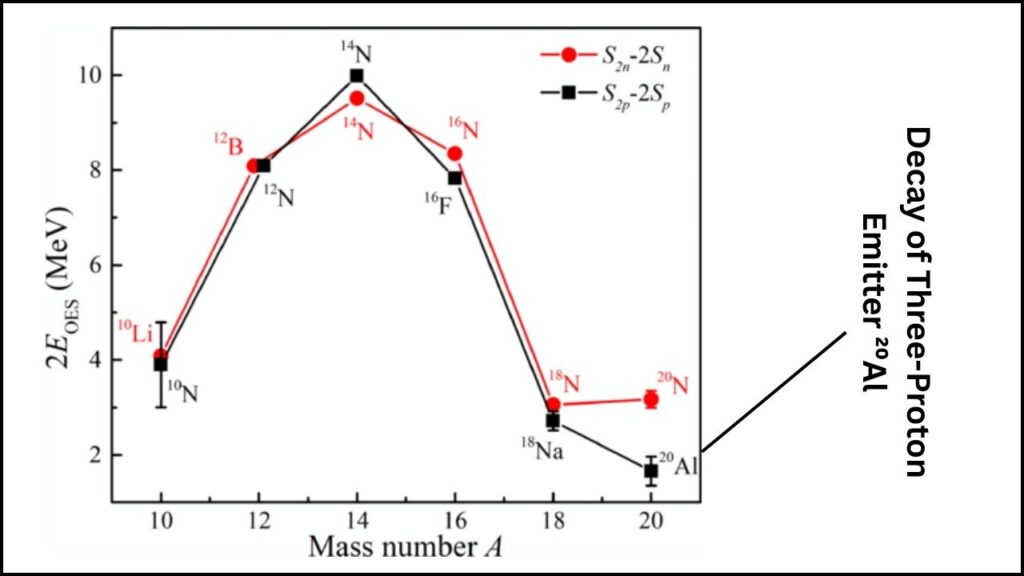
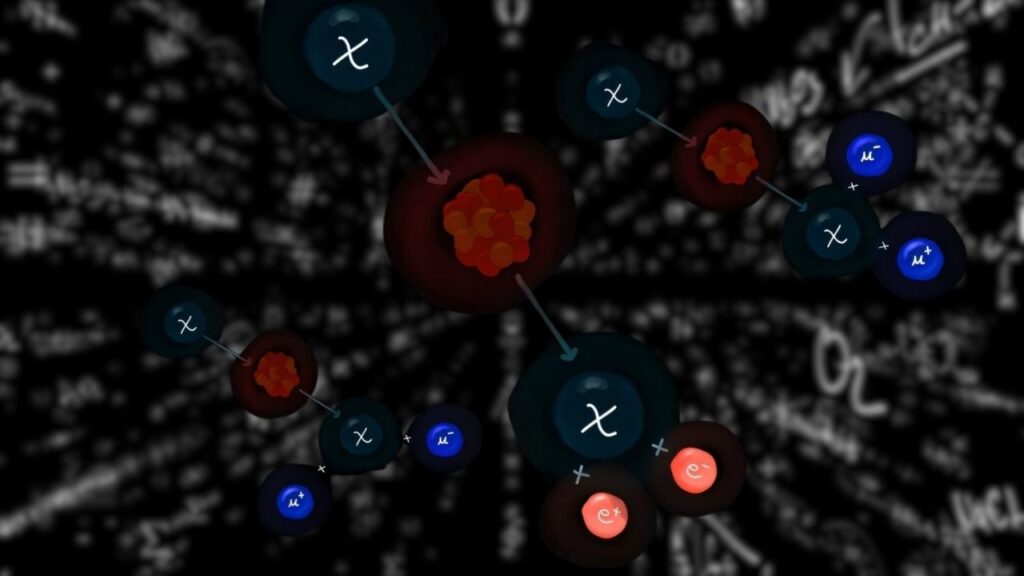
To confirm this, researchers measured the directions and energies of the particles released during its decay. Aluminum-20 first emits one proton, turning into magnesium-19. But magnesium-19 is also too light to hold together, so it immediately ejects two more protons. This three-proton emission is an incredibly rare form of nuclear decay.
Why Is Three-Proton Emission So Rare?
Nuclear decay usually involves emitting one particle at a time—an alpha particle, a beta particle, or a neutron. Proton emission is already uncommon. But three-proton emission is extremely rare, almost like a nucleus “exploding” from the inside.
This discovery is the first time scientists have seen an isotope decay in this way, especially where the first product of decay is also itself proton-unstable. It gives physicists a brand new way to study nuclear forces and what holds nuclei together—or what makes them fall apart.
What Does This Mean for Nuclear Physics?
Nuclei are held together by a delicate balance of forces between protons and neutrons. The “proton drip line” is the boundary beyond which adding more protons makes a nucleus too unstable to exist. Aluminum-20 is far beyond this line, so its behavior challenges existing nuclear models.
The decay energy of aluminum-20 is lower than expected, and its “mirror nucleus,” neon-20, behaves differently, too. This suggests a breakdown in “isospin symmetry,” a theoretical idea that protons and neutrons are similar in certain ways. By studying aluminum-20, scientists can improve their models and understand the limits of nuclear existence.
What Are the Practical Applications?
Aluminum-20 itself is too short-lived for practical uses. But the research behind its discovery has broader value:
- Fundamental Science: Exploring the edges of the periodic table helps us understand the behavior of matter under extreme conditions, which is important for understanding the universe.
- Astrophysics: Rare nuclear reactions help explain how elements form in stars and supernovae.
- Technology: The advanced detectors and analysis methods developed for such experiments can lead to new tools in medicine, energy, and national security.
How Is Research Like This Done?
Discovering a new isotope like aluminum-20 is a multi-step process:
- Design the Experiment: Teams use nuclear models to predict what new isotopes might exist and how to create them.
- Build the Equipment: Facilities like GSI use powerful accelerators and sensitive detectors to produce and measure these ultra-short-lived nuclei.
- Collect Data: Scientists analyze the signals from the detectors to spot rare events.
- Publish Results: Once confirmed, findings are shared in scientific journals so other experts can review and build on the work.
Aluminum Ion-Based Clock Breaks Record With 19-Decimal Precision
Scientists Discover Hidden Aluminum Sites That Supercharge Zeolite Catalysis
Ultrasound Technique Accurately Detects Defects in Aluminum Manufacturing
Frequently Asked Questions
What is aluminum-20?
Aluminum-20 is the lightest known isotope of aluminum. It has only seven neutrons and is extremely unstable, existing for less than a millionth of a second.
How was it discovered?
An international team created aluminum-20 by accelerating nuclei to high speeds and measuring their decay at a specialized laboratory in Germany.
Why is three-proton emission important?
This rare decay mode helps scientists test and refine nuclear models, especially for nuclei at the very edge of stability.
What is the proton drip line?
It’s the boundary beyond which adding more protons makes a nucleus so unstable it immediately ejects protons.
Could aluminum-20 ever be used in real life?
No—it’s too unstable for practical applications, but the research advances nuclear science and technology.
Why should I care about discoveries like this?
Pushing the boundaries of science often leads to unexpected breakthroughs, new technologies, and a deeper understanding of the universe.