In the world of science and sustainability, few innovations have sparked as much excitement as the breakthrough catalyst that efficiently converts CO₂ into methanol with high precision. This development is more than just a laboratory success—it’s a potential game-changer for climate action, clean energy, and industry. Whether you’re a curious student, a professional in the field, or just someone who cares about the planet, understanding this breakthrough can help you see the future of green technology in a whole new light.
Table of Contents
Breakthrough Catalyst Efficiently Converts CO₂ Into Methanol
| Feature/Fact | Details |
|---|---|
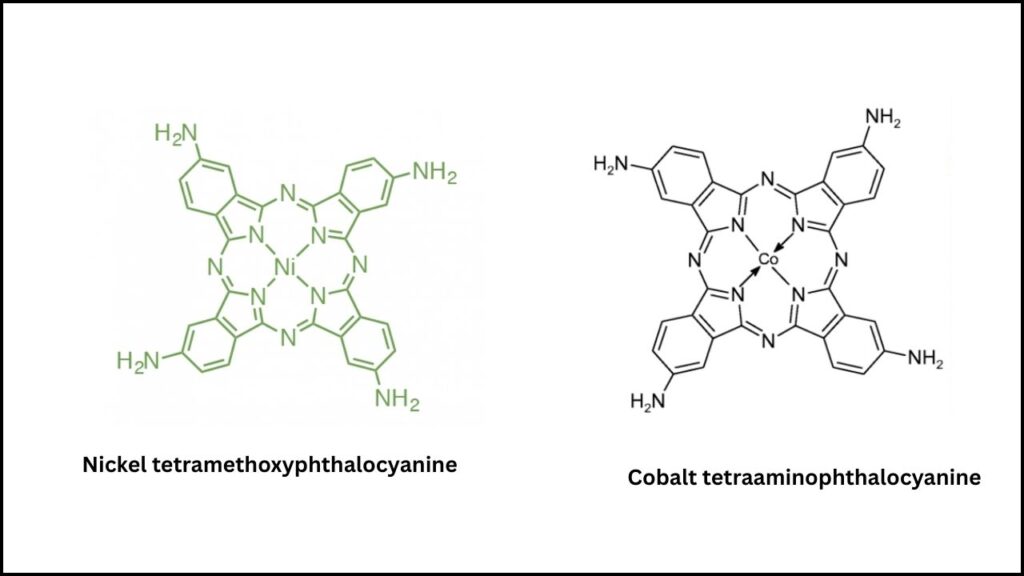
| Breakthrough Catalyst | Dual-site system: nickel tetramethoxyphthalocyanine (NiPc-OCH₃) and cobalt tetraaminophthalocyanine (CoPc-NH₂) |
| Efficiency Improvement | Up to 50% CO₂-to-methanol conversion (about 66% higher than previous methods) |
| Selectivity | Over 91% selectivity for methanol in optimized systems |
| Industrial Impact | Scalable for recycling industrial CO₂ emissions into renewable fuel |
| Professional Pathways | Careers in green chemistry, catalysis research, renewable fuels, and industrial sustainability |
| Official Resource | Nature Nanotechnology |
The development of a breakthrough catalyst that efficiently converts CO₂ into methanol with high precision represents a major leap forward in the quest for sustainable energy and climate solutions. By making it possible to recycle industrial emissions into valuable fuel, this technology has the potential to reshape industries, create new jobs, and help the world meet its climate goals.
If you’re interested in the future of green technology, now is the time to learn more, get involved, and be part of the solution.
Why Is CO₂-to-Methanol Conversion So Important?
Let’s start with the basics. CO₂-to-methanol conversion is the process of turning carbon dioxide—a major greenhouse gas—into methanol, a liquid that can be used as fuel or as a building block for many everyday products.
Why does this matter? Because:
- It helps reduce the amount of CO₂ in the atmosphere, which is key to fighting climate change.
- It creates a renewable fuel that can power cars, ships, and even airplanes.
- Methanol can be used to make plastics, paints, and other chemicals, offering a sustainable alternative to fossil-based sources.
Imagine a world where the exhaust from factories is captured and transformed into something valuable, instead of polluting the air. That’s the promise of this technology.
The Science Behind the Breakthrough
The Challenge: Turning a Problem Into a Solution
Converting CO₂ into methanol isn’t new—but doing it efficiently and selectively has always been a challenge. Traditional methods often require high temperatures and pressures, and they tend to produce unwanted byproducts like carbon monoxide (CO) or methane, which makes the process less efficient and more expensive.
The Solution: The Dual-Site Catalyst
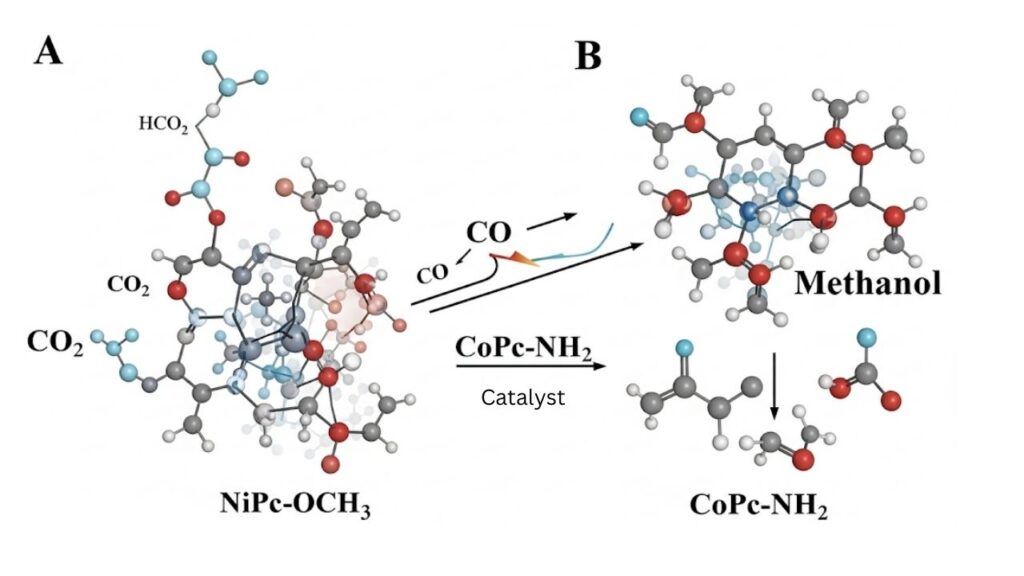
The latest breakthrough involves a dual-site catalyst system. This means two different catalysts are used together, each performing a specific role in the conversion process:
- Nickel tetramethoxyphthalocyanine (NiPc-OCH₃): This catalyst is responsible for the first step—converting CO₂ into carbon monoxide (CO).
- Cobalt tetraaminophthalocyanine (CoPc-NH₂): This catalyst takes over next, turning the CO into methanol.
Think of it like a relay race: the first runner (NiPc-OCH₃) passes the baton (CO) to the second runner (CoPc-NH₂), who finishes the race by producing methanol. This teamwork makes the process much more efficient and precise.
How Does the Catalyst Actually Work?
- Step 1: CO₂ molecules are captured and brought into contact with the NiPc-OCH₃ catalyst, which helps break the strong bonds in CO₂ and transforms it into CO.
- Step 2: The CO produced doesn’t just float away. Instead, it “spills over” to the nearby CoPc-NH₂ catalyst, where it’s further converted into methanol.
- Step 3: The methanol is collected and can be used as a fuel or chemical feedstock.
This dual-site approach mimics the way nature often solves complex problems—by breaking them into smaller, manageable steps, each handled by a specialist.
What Makes This Breakthrough So Valuable?
Higher Efficiency and Selectivity
One of the biggest achievements of this new catalyst system is its efficiency. Previous methods managed to convert about 30% of CO₂ into methanol. With the new approach, that number jumps to up to 50%. That’s a massive improvement, especially when you consider the scale at which industrial CO₂ emissions occur.
Selectivity is another key metric. In chemical reactions, selectivity means producing mostly what you want (in this case, methanol) and very little of what you don’t (like CO or methane). The new catalyst achieves over 91% selectivity for methanol, which means almost all the CO₂ is turned into the desired product.
Real-Time Monitoring for Precision
To ensure the process works as intended, scientists use advanced tools like sum-frequency generation vibrational spectroscopy (SFG-VS) and X-ray photoelectron spectroscopy (XPS). These allow them to watch the reaction as it happens, confirming that CO really does move from one site to the other before becoming methanol. This level of control is essential for scaling up the process and ensuring consistent results.
Designed for Industrial Use
Perhaps most importantly, the new catalyst system is designed to be scalable. That means it can be used in real-world factories, not just in laboratory experiments. Industries that emit large amounts of CO₂—like steel, cement, and chemical manufacturing—can potentially install this technology to capture their emissions and turn them into valuable methanol.
How Does CO₂-to-Methanol Conversion Work? A Step-by-Step Guide
1. CO₂ Capture
CO₂ is collected from industrial smokestacks or directly from the air using special filters or membranes. This is the first step in many carbon capture and utilization (CCU) strategies.
2. Feeding CO₂ to the Catalyst
The captured CO₂ is sent into a reactor containing the dual-site catalyst system.
3. First Reaction: CO₂ to CO
At the nickel site, CO₂ is converted into carbon monoxide (CO). This step is crucial because CO is much easier to turn into methanol than CO₂.
4. Spillover to the Second Site
The CO produced at the nickel site moves to the cobalt site. This “spillover” is what makes the dual-site system so effective.
5. Second Reaction: CO to Methanol
At the cobalt site, the CO is converted into methanol (CH₃OH).
6. Methanol Collection
The methanol is separated from the reaction mixture and collected for use as a fuel or chemical feedstock.
Real-World Example: How a Factory Could Use This Technology
Let’s imagine a large steel factory. Traditionally, this factory emits thousands of tons of CO₂ every year. With the new catalyst system:
- The factory installs a CO₂ capture system on its smokestacks.
- The captured CO₂ is piped into a reactor filled with the dual-site catalyst.
- Inside the reactor, CO₂ is efficiently converted into methanol.
- The methanol is collected and can be sold as a fuel, used to make chemicals, or even power the factory itself.
- The factory dramatically reduces its carbon footprint and creates a new revenue stream.
This is not just a vision for the future—pilot projects are already exploring these approaches in various industries worldwide.
Career and Professional Opportunities
The rise of efficient CO₂-to-methanol conversion opens up exciting new career paths:
- Green Chemistry: Professionals who understand catalysis, reaction engineering, and sustainable processes are in high demand.
- Industrial Engineering: Designing and operating large-scale reactors for CO₂ conversion requires skilled engineers.
- Environmental Science: Experts who can analyze the environmental impact and optimize CO₂ capture and utilization will play a key role.
- Policy and Regulation: As governments set stricter emissions targets, there’s a growing need for professionals who can bridge science, technology, and policy.
If you’re studying or working in chemistry, engineering, or environmental science, now is a great time to get involved in this rapidly growing field.
Cobalt-Based Catalyst Offers an Effective Alternative to Precious Metals
Hydrogen Fuel at Half the Cost? New Catalyst from South Korea
Precisely Placed Platinum Atoms Boost Catalyst Efficiency and Speed
FAQs About Breakthrough Catalyst Efficiently Converts CO₂ Into Methanol
What is methanol, and why is it important?
Methanol is a simple alcohol used as a fuel, solvent, and building block for making plastics, paints, and other chemicals. It’s important because it can replace fossil fuels in many applications and can be produced renewably from CO₂.
How does this catalyst help fight climate change?
By turning CO₂—a major greenhouse gas—into methanol, the process reduces emissions and creates a valuable product. This helps close the carbon loop and supports a more sustainable economy.
Is this technology ready for commercial use?
The new catalyst has shown promising results in laboratory and pilot-scale studies. While more work is needed to scale it up, industries are already exploring commercial applications.
Can this process use CO₂ from the air?
Yes, the technology can use CO₂ captured from industrial sources or directly from the atmosphere, making it versatile for many applications.
How does this compare to traditional methods?
Traditional methods are less efficient and often produce unwanted byproducts. The new dual-site catalyst is significantly more efficient and selective, making it a superior option for sustainable methanol production.
Practical Advice: How to Get Involved
- Students: Focus on studies in chemistry, chemical engineering, materials science, or environmental engineering. Look for internships or research projects related to catalysis or carbon capture.
- Professionals: Stay updated with the latest advancements in green chemistry and sustainable technologies. Attend industry conferences and join professional associations.
- Industry Leaders: Assess your company’s CO₂ emissions and explore pilot projects using advanced catalyst systems. Collaborate with universities and research institutes to stay at the forefront of innovation.