CAR-T Cancer Therapy: A child who underwent CAR-T cell therapy—a revolutionary immunotherapy treatment—for a deadly childhood cancer has remained disease-free for 18 years, marking one of the longest and most remarkable remissions ever reported in solid tumor treatment using CAR-T cells. This case has helped pave the way for broader use of CAR-T therapies and renewed hope for patients with cancers once considered untreatable.
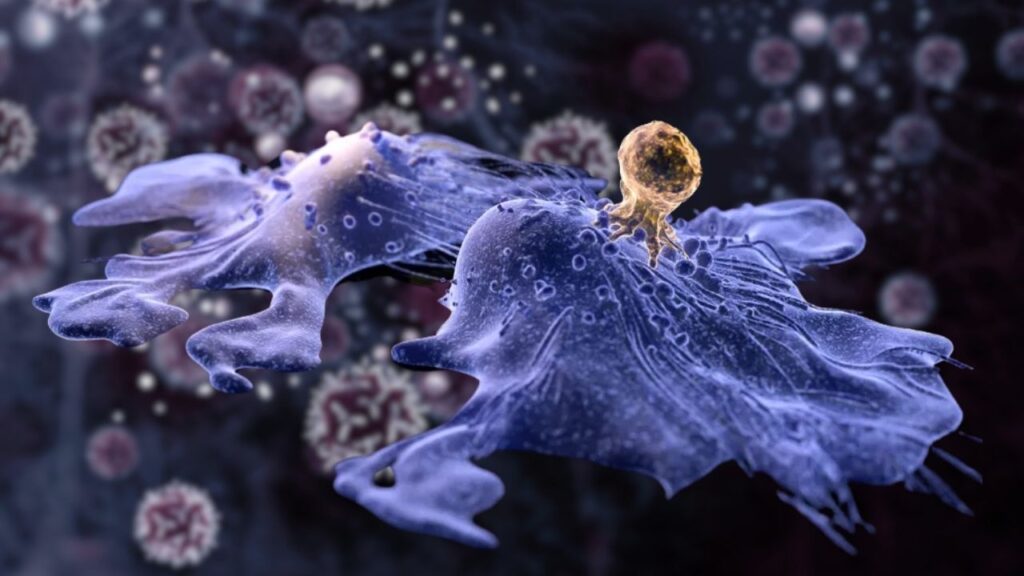
The patient, diagnosed at age 6 with neuroblastoma, an aggressive pediatric cancer affecting nerve tissue, participated in a pioneering clinical trial at the Children’s Hospital of Philadelphia (CHOP) in 2005. Since then, she has lived a healthy life into adulthood, including having two healthy children—a testament to the power and promise of CAR-T cell therapy.
The extraordinary case of a child treated with CAR-T therapy for neuroblastoma who remains cancer-free 18 years later offers hope and valuable lessons for cancer treatment worldwide. This success illustrates the potential of immunotherapy to not only extend life but to restore it fully.
While challenges remain for CAR-T treatment of solid tumors, ongoing research, new trials, and technological improvements are making what once seemed impossible increasingly achievable.
Table of Contents
What Is CAR-T Cell Therapy and Why Is It Important?
CAR-T cell therapy (Chimeric Antigen Receptor T-cell therapy) is a form of personalized immunotherapy that involves modifying a patient’s own immune cells to recognize and attack cancer cells. Unlike traditional treatments like chemotherapy and radiation, which kill both cancerous and healthy cells, CAR-T therapy is designed to be a targeted treatment with potentially fewer side effects.
The process involves:
- Extracting T cells from the patient’s blood.
- Using genetic engineering to add a chimeric antigen receptor (CAR) to the surface of these T cells.
- Multiplying the modified cells in the lab.
- Infusing the enhanced T cells back into the patient.
Once inside the body, these CAR-T cells identify and destroy cancer cells expressing specific markers.
The FDA has approved CAR-T therapy for several blood cancers:
- B-cell acute lymphoblastic leukemia (ALL)
- Diffuse large B-cell lymphoma (DLBCL)
- Multiple myeloma
However, success with solid tumors remains limited, making this 18-year remission case particularly groundbreaking.
Understanding Neuroblastoma and Its Challenges
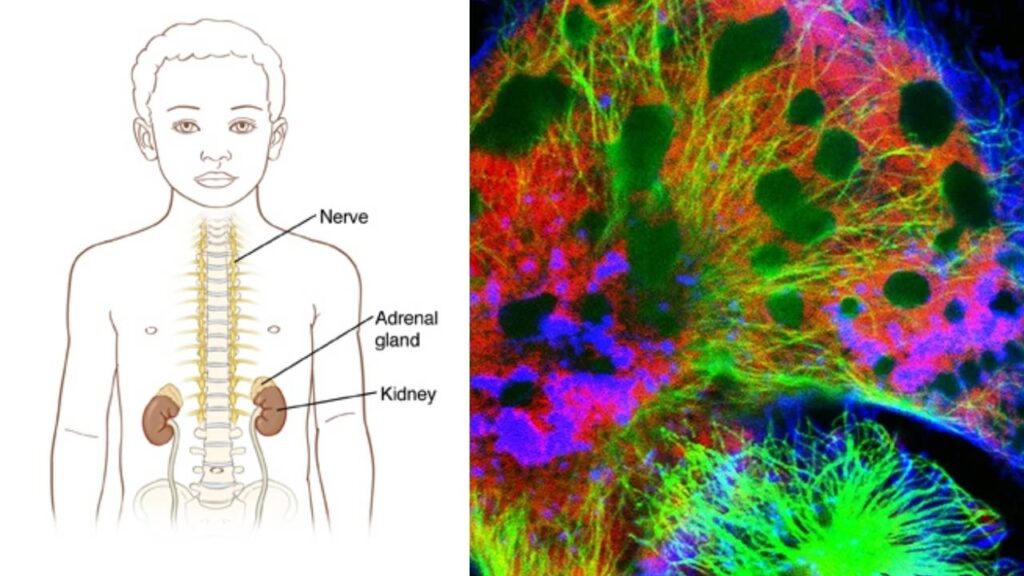
Neuroblastoma is a cancer that arises from immature nerve cells called neuroblasts, usually in the adrenal glands or along the spine. It most commonly affects children under 5 years old and accounts for roughly 6-10% of all childhood cancers, but it is responsible for about 15% of cancer-related deaths in children.
Why is neuroblastoma so difficult to treat?
- Aggressiveness: Neuroblastoma can grow quickly and spread to other parts of the body.
- Heterogeneity: Tumors have diverse genetic profiles making uniform treatment difficult.
- Resistance: It can resist chemotherapy and radiation.
- Tumor microenvironment: The surrounding tissue can suppress immune attack and promote tumor growth.
Conventional treatments include surgery, chemotherapy, radiation, stem cell transplant, and immunotherapy with anti-GD2 antibodies. But long-term survival rates for high-risk neuroblastoma remain around 50%, demonstrating the need for new therapies.
The Breakthrough: CAR-T Targeting GD2 in Neuroblastoma
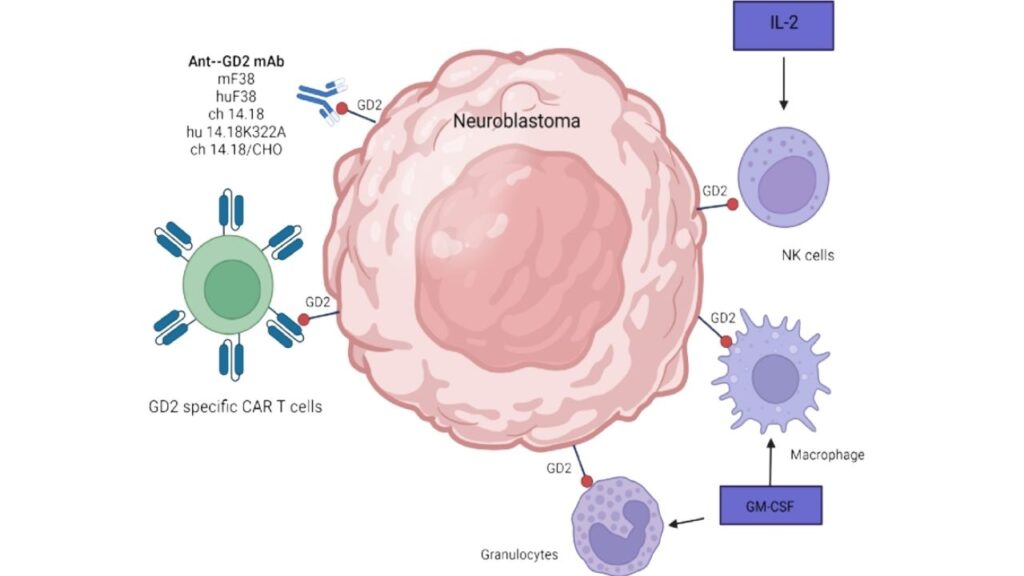
CAR-T therapy’s success in blood cancers encouraged researchers to explore its application in neuroblastoma. The target? A molecule called GD2, which is highly expressed on neuroblastoma cells but minimally found on healthy cells, making it an ideal target.
How did the therapy work?
- The patient’s T cells were modified to express a CAR that binds specifically to GD2.
- These engineered T cells were expanded and infused back into the patient.
- The CAR-T cells located and attacked neuroblastoma cells, sparing most normal tissue.
This trial was among the first to use CAR-T therapy for solid tumors, which are traditionally harder to treat due to the protective tumor microenvironment and physical barriers.
Detailed Timeline of the Patient’s Journey
- Diagnosis (2005): The patient was diagnosed with high-risk neuroblastoma.
- Enrollment in Trial: After standard therapies failed, she was enrolled in the CAR-T trial led by Dr. Stephan Grupp and Dr. Carl June at CHOP.
- Treatment: She received CAR-T cells targeting GD2.
- Immediate Response: The cancer showed significant reduction within months.
- Long-Term Follow-Up: Over the next 18 years, the patient remained in complete remission.
- Current Status: As of 2025, she leads a normal adult life with no detectable cancer, normal immune function, and two healthy children.
Why Is This Remission So Significant?
CAR-T therapy has shown unprecedented results in blood cancers, but solid tumors have remained a challenge due to:
- Tumor microenvironment: Solid tumors create a suppressive environment that limits T cell infiltration and survival.
- Antigen heterogeneity: Variability in tumor markers makes targeting difficult.
- T cell exhaustion: Immune cells can become “tired” and less effective.
The fact that this patient remains disease-free after nearly two decades demonstrates:
- The potential for CAR-T to achieve durable remission in solid tumors.
- Proof that genetically modified T cells can persist long-term in the body.
- The possibility of immune memory that prevents relapse.
Current Status of CAR-T in Solid Tumor Research
Despite this breakthrough, challenges remain for widespread CAR-T use in solid tumors:
- Limited infiltration: Strategies like combining CAR-T with checkpoint inhibitors or radiation are under study to help immune cells penetrate tumors.
- Safety concerns: Targeting tumor-specific antigens is vital to avoid damaging healthy tissues.
- Cost and complexity: CAR-T manufacturing is expensive and labor-intensive.
Ongoing clinical trials are testing CAR-T therapy for solid tumors including:
- Glioblastoma
- Pancreatic cancer
- Ovarian cancer
- Melanoma
According to ClinicalTrials.gov, over 1,000 CAR-T trials are active worldwide as of 2025, highlighting global investment in this therapy’s future.
Practical Advice for Patients and Families
If you or a loved one is considering CAR-T therapy, here are some steps and considerations:
1. Consult a Specialist
Seek care from a cancer center with CAR-T expertise. Not all hospitals offer this therapy.
2. Understand Eligibility
CAR-T is approved primarily for certain blood cancers, but many clinical trials exist for other cancers. Eligibility depends on cancer type, prior treatments, and overall health.
3. Discuss Risks and Benefits
Side effects can include:
- Cytokine Release Syndrome (CRS): a systemic inflammatory response.
- Neurological symptoms like confusion.
- Low blood counts and infection risk.
4. Plan for Follow-Up
Long-term monitoring is critical. Remission does not guarantee cure, but ongoing surveillance can catch relapse early.
5. Explore Clinical Trials
New trials expand access to CAR-T. Visit ClinicalTrials.gov for listings.
Expert Commentary
“This case is a proof of principle that immune cells can be harnessed against solid tumors and induce long-lasting remissions. It offers hope but also reminds us of the complexity of cancer immunotherapy,” said Dr. Carl June, pioneer in CAR-T therapy.
Dr. Stephan Grupp from CHOP added, “Seeing a child grow into a healthy adult and start a family after such aggressive cancer gives profound meaning to our work. It shows that scientific innovation translates into real lives saved.”
AI-Driven Models Accelerate Molecular and Materials Discovery in Biomedical Research
Superconducting Diode Bridge Efficiently Converts AC to DC for Quantum Circuits
China Develops High-Resolution Laser Capable of Reading Tiny Text from Over a Kilometer Away
FAQs About CAR-T Cancer Therapy
How long does CAR-T therapy take?
The entire process, including cell collection, modification, and infusion, usually takes 2-4 weeks.
What is the cost of CAR-T therapy?
Costs can range from $350,000 to $500,000 depending on the treatment and location, but many insurance plans and Medicare may cover FDA-approved indications.
Can CAR-T therapy be repeated?
In some cases, yes, but doctors assess risks and benefits carefully.
Are there any lifestyle changes after treatment?
Patients are advised to maintain healthy habits, avoid infections, and attend regular follow-ups but can typically resume normal activities.
How effective is CAR-T therapy in pediatric cancers?
For blood cancers like ALL, remission rates exceed 80% in children. Solid tumor success rates are lower but improving.