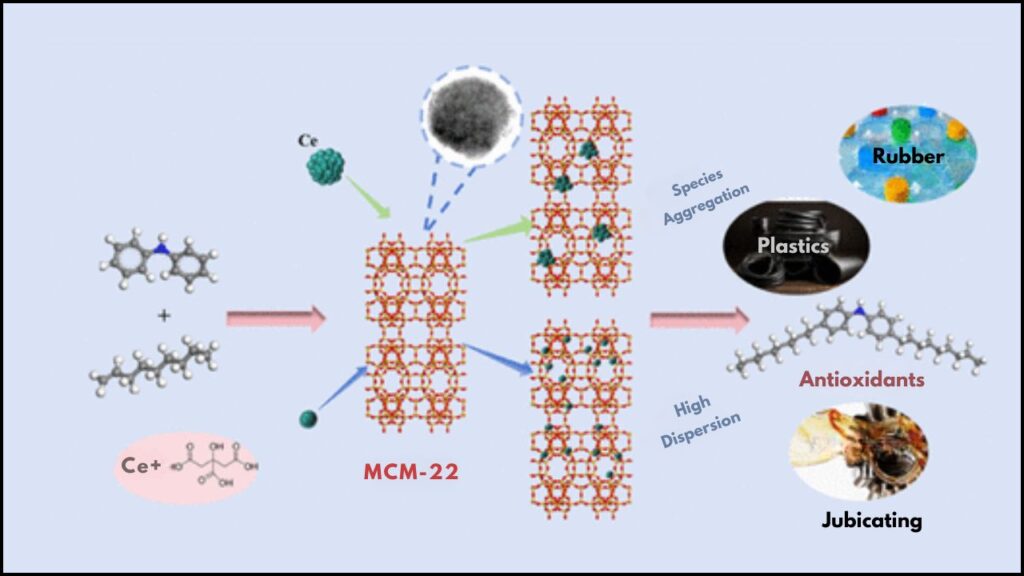
Diphenylamine alkylation is an essential chemical process widely used to produce antioxidants, stabilizers, and various industrial chemicals. Recently, a breakthrough catalyst has emerged that greatly enhances this process—citric acid-dispersed cerium (Ce) on MCM-22 zeolite. This innovative catalyst system improves efficiency, precision, and environmental friendliness in diphenylamine alkylation.
In this article, we’ll delve into this topic in an easy-to-understand way—yet rich with valuable insights for professionals. You’ll get context about diphenylamine alkylation, the special features of this catalyst, practical advice on how it works, and why it’s a game-changer.
Table of Contents
Catalysts for Diphenylamine Alkylation
| Key Aspect | Details |
|---|---|
| Reaction | Alkylation of diphenylamine (DPA) with alkenes |
| Catalyst | Cerium (Ce) highly dispersed using citric acid on MCM-22 zeolite |
| Catalyst Benefits | Enhanced dispersion, higher activity, improved selectivity, and longer durability |
| Typical Reaction Conditions | Liquid-phase alkylation at 110–200°C |
| Industry Use | Production of antioxidants and stabilizers for rubber, lubricants, plastics |
| Performance Metrics | Up to 98% conversion of DPA, high selectivity for dialkylated products |
| Environmental Impact | Reduced catalyst poisoning and safer, greener chemistry |
| Official Reference | East China University of Science and Technology |
The development of a citric acid-dispersed cerium catalyst on MCM-22 zeolite marks a breakthrough in diphenylamine alkylation. This catalyst enhances chemical conversion, selectivity, and durability while promoting more sustainable and cost-effective industrial processes. Whether you’re a chemical engineer, researcher, or industry professional, understanding this catalyst’s advantages and application offers a pathway to improved antioxidant and stabilizer production.
Understanding Diphenylamine Alkylation
What is Diphenylamine Alkylation?
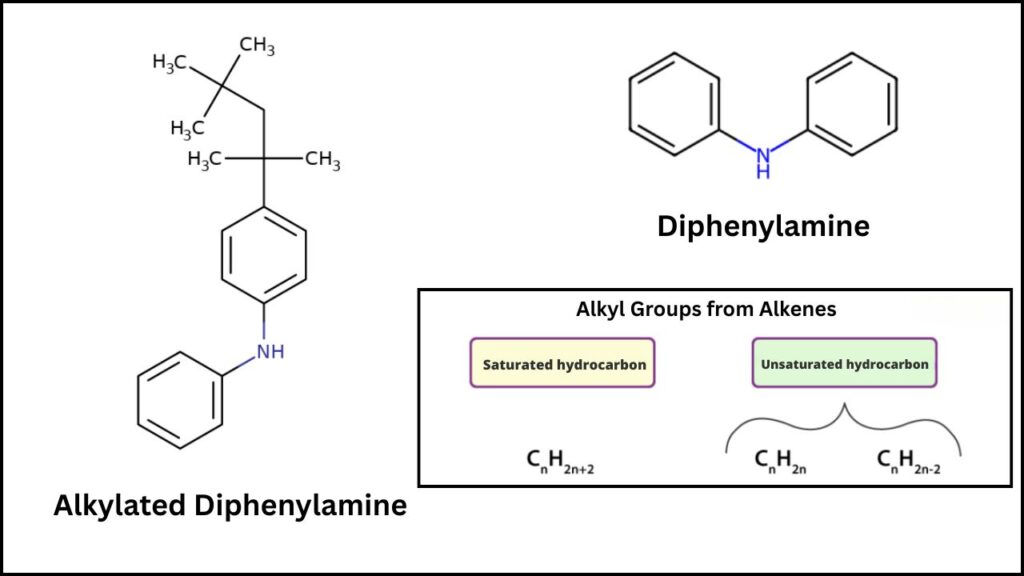
At its core, diphenylamine alkylation is a chemical reaction where diphenylamine (DPA) molecules are bonded with alkyl groups from alkenes (unsaturated hydrocarbons). This produces alkylated diphenylamines, compounds widely used as:
- Antioxidants in rubber and plastics to prevent degradation
- Lubricant additives to protect engines
- Stabilizers for fuels and oils
This reaction needs a catalyst to proceed efficiently—traditionally strong acids or clay-based materials, which sometimes suffer from limited activity or selectivity.
Why Is Alkylation Important?
Alkylated diphenylamines improve durability and performance in many products. For example, antioxidants derived from alkylated DPAs prolong the life of automotive tires, plastic materials, and engine oils by preventing oxidation damage.
What Makes the Citric Acid-Dispersed Ce on MCM-22 Catalyst Special?
The Role of Cerium (Ce)

Cerium is a rare earth metal known for its versatile catalytic properties. When finely dispersed on a support material, cerium enhances chemical reactions by facilitating electron transfer and activating reactants more efficiently.
MCM-22 Zeolite: The Ideal Support
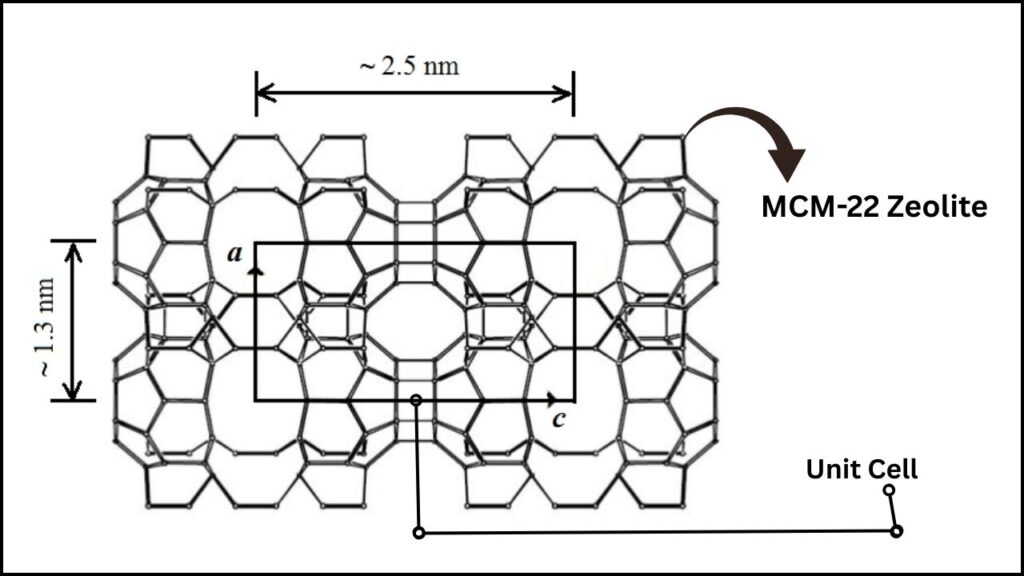
MCM-22 is a type of molecular sieve (zeolite) with a unique porous structure and acidity. It provides:
- A large surface area
- Unique pore structure for selective molecular access
- Acid sites that help in the alkylation mechanism
The Magic of Citric Acid Dispersion
Citric acid plays a dual role:
- It acts as a chelating agent, helping distribute cerium ions evenly over the surface of the MCM-22 zeolite.
- Prevents cerium particle agglomeration, ensuring high dispersion and therefore more active sites for the reaction.
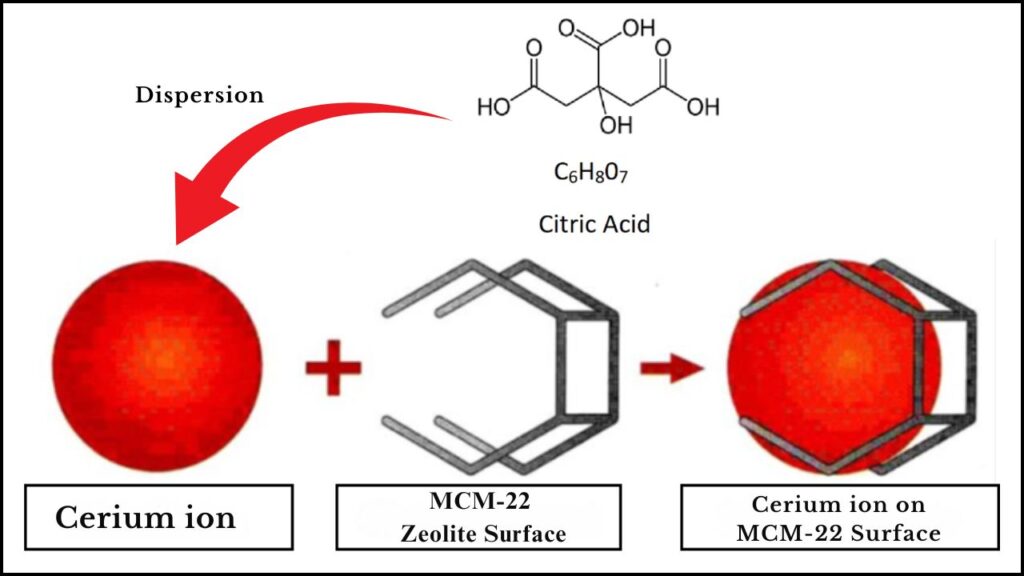
This results in a highly dispersed cerium catalyst on MCM-22, which exhibits superior catalytic activity, selectivity, and longevity compared to traditional catalysts.
How Does the Catalyst Work?
The alkylation reaction mechanism involves several steps:
- Protonation of the Olefin: The acidic sites on MCM-22 activate the alkene molecule by protonation, creating an electrophile.
- Electrophilic Attack: The protonated alkene reacts with diphenylamine, forming an alkylated intermediate.
- Catalyst Facilitation: Cerium helps stabilize reaction intermediates and lowers energy barriers, improving conversion efficiency and selectivity.
- Product Formation: After rearrangements, the dialkylated diphenylamine product is formed and released.
Thanks to the citric acid-induced dispersion of cerium, the catalyst can achieve up to 98% conversion of diphenylamine under optimal conditions (temperature range: 110–200°C) while maintaining high selectivity toward desired products.
Practical Guide to Using the Catalyst
Step 1: Catalyst Preparation
- Disperse cerium precursor salts on MCM-22 using citric acid as a dispersing agent.
- Heat and treat the mixture to stabilize the cerium dispersion on the zeolite surface.
Step 2: Setting Reaction Conditions
- Use liquid phase alkylation of DPA with alkenes (e.g., isobutylene or nonene).
- Maintain temperature between 110°C and 200°C, with 160°C as an optimum compromise for conversion and selectivity.
- Keep reaction time consistent for full conversion (~300 minutes).
Step 3: Monitoring and Optimization
- Track diphenylamine conversion to ensure catalyst performance.
- Control alkene chain length as longer chains reduce conversion rates.
- Avoid catalyst poisoning by minimizing by-products like cracking products.
Step 4: Post-Reaction Handling
- Separate and purify the dialkylated diphenylamine products.
- Regenerate or replace catalyst as needed based on longevity studies.
Why This Breakthrough Matters for Industry
- Higher Efficiency: Faster, more complete conversion saves time and materials.
- Improved Product Quality: Higher selectivity reduces impurities and enhances product performance.
- Greener Process: Reduced catalyst deactivation lowers waste, and citric acid usage is more environmentally friendly.
- Economic Benefits: Longer catalyst life and better yields cut down operational costs.
For industries relying on antioxidants and stabilizers, this catalyst system represents a significant advance.
New Carbon Catalyst Could Revolutionize Biodiesel — No Metals Needed
Engineered Ruthenium Catalysts: A Sustainable Revolution for Hydrogen and Ammonia
New Triple-Layer Catalyst Boosts Green Hydrogen Output by 800%—A Game-Changer for Clean Energy
FAQs About Catalysts for Diphenylamine Alkylation
What is the main advantage of using cerium on MCM-22?
Cerium improves the catalytic activity by providing additional reactive sites and electron transfer capabilities when highly dispersed on the MCM-22 zeolite, leading to higher conversion and selectivity.
Why is citric acid important in this catalyst?
Citric acid helps distribute cerium evenly, preventing particle clumping, which in turn ensures more active sites are available for the alkylation reaction.
What types of alkenes can be used for diphenylamine alkylation?
Common alkenes include isobutylene, nonene, and diisobutylene. Reactivity decreases with longer alkyl chains, so shorter chains like isobutylene are preferred for higher conversion.
At what temperature is the reaction typically carried out?
The reaction usually occurs between 110°C and 200°C, with 160°C being optimal to balance conversion efficiency and product selectivity.
Is this catalyst environmentally friendly?
Yes. The use of citric acid as a dispersant and the high efficiency of the catalyst reduce waste and the need for harsh chemicals, making the process greener.