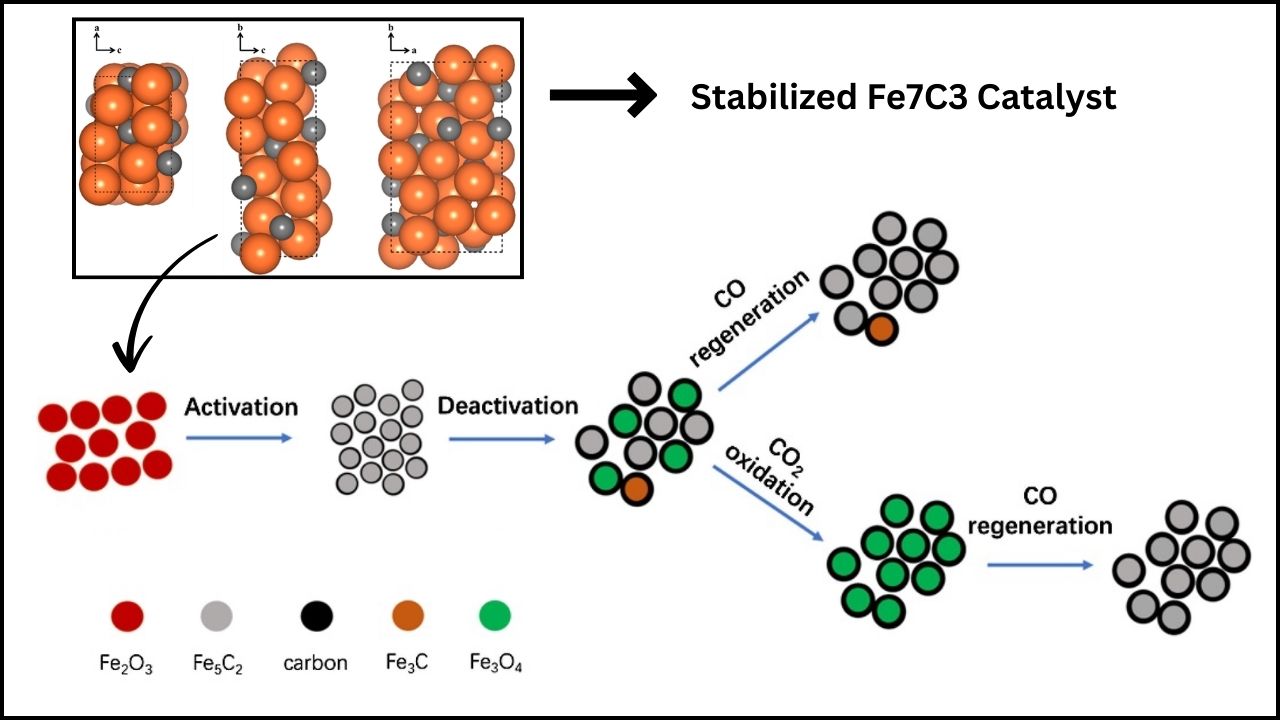
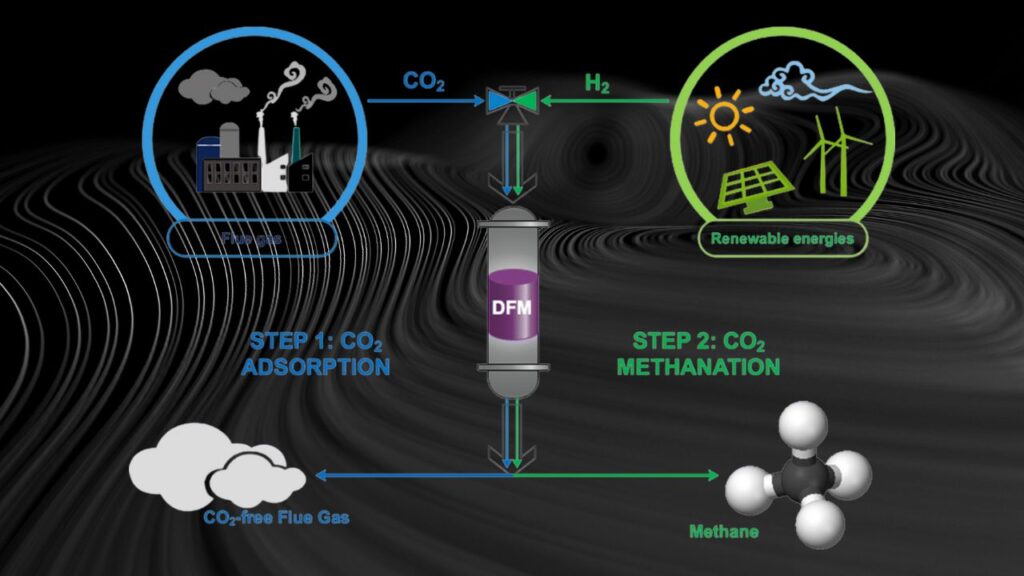
CO₂ to Methane Conversion: The conversion of carbon dioxide (CO₂) to methane (CH₄) has taken a significant leap forward thanks to cutting-edge research focusing on nickel (Ni) nanoparticles. Scientists have discovered that by fine-tuning the shape and size of nickel nanoparticles, they can dramatically improve the efficiency of this conversion process. This breakthrough not only brings us closer to sustainable fuel production but also opens up new possibilities for reducing greenhouse gas emissions.
This article dives into the science behind this innovation, explaining the importance of nanoparticle shape in catalysis, its practical applications, and how this discovery could impact energy industries and climate change mitigation. Whether you’re a student, a researcher, or simply curious about green technologies, this guide will help you understand the essentials of CO₂ methanation and why nickel nanoparticles are at the center of this exciting development.
CO₂ to Methane Conversion
| Topic | Details |
|---|---|
| Core Discovery | Shape and size of Ni nanoparticles influence CO₂ to methane catalytic conversion efficiency. |
| Reaction Involved | Sabatier reaction: CO₂ + 4H₂ → CH₄ + 2H₂O |
| Research Institution | Politecnico di Milano |
| Publication | ACS Catalysis (2024) |
| Catalyst Advantage | Increased activity and selectivity by optimizing nanoparticle morphology |
| Environmental Impact | Potential to reduce greenhouse gases by recycling CO₂ into useful fuels |
| Industrial Applications | Fuel synthesis, ammonia production, Fischer–Tropsch synthesis |
| Official Research Link | ACS Catalysis Article |
The breakthrough in tweaking nickel nanoparticle shape to unlock CO₂ to methane conversion is a promising step toward sustainable fuel production and climate change mitigation. By understanding and harnessing the power of nanostructure engineering, scientists and industries can make CO₂ methanation more efficient, practical, and environmentally friendly. This discovery underscores the importance of nanoscale design in catalytic chemistry and opens up new pathways for green energy innovation.
Understanding CO₂ to Methane Conversion
What Is CO₂ Methanation?
CO₂ methanation is a chemical reaction where carbon dioxide reacts with hydrogen gas to produce methane and water. This process is commonly referred to as the Sabatier reaction, named after Paul Sabatier, who discovered it in the early 1900s.
The simplified chemical equation is: CO2+4H2→CH4+2H2O\text{CO}_2 + 4\text{H}_2 \rightarrow \text{CH}_4 + 2\text{H}_2\text{O}CO2+4H2→CH4+2H2O
Methane produced through this method can be used as a clean fuel or as a feedstock for various chemical industries.
Why Convert CO₂ to Methane?
Carbon dioxide is a major greenhouse gas responsible for climate change. Finding ways to reduce atmospheric CO₂ or recycle it into valuable products helps combat global warming. Methane, on the other hand, is a clean-burning fuel that can substitute fossil fuels.
By converting CO₂ into methane, industries can:
- Lower their carbon footprint.
- Create renewable energy sources.
- Develop sustainable chemical manufacturing processes.
The Role of Nickel Nanoparticles in CO₂ Methanation
Why Nickel?
Nickel is a widely used catalyst in CO₂ methanation because of its high activity and availability compared to precious metals like ruthenium or rhodium. However, not all nickel catalysts perform equally.
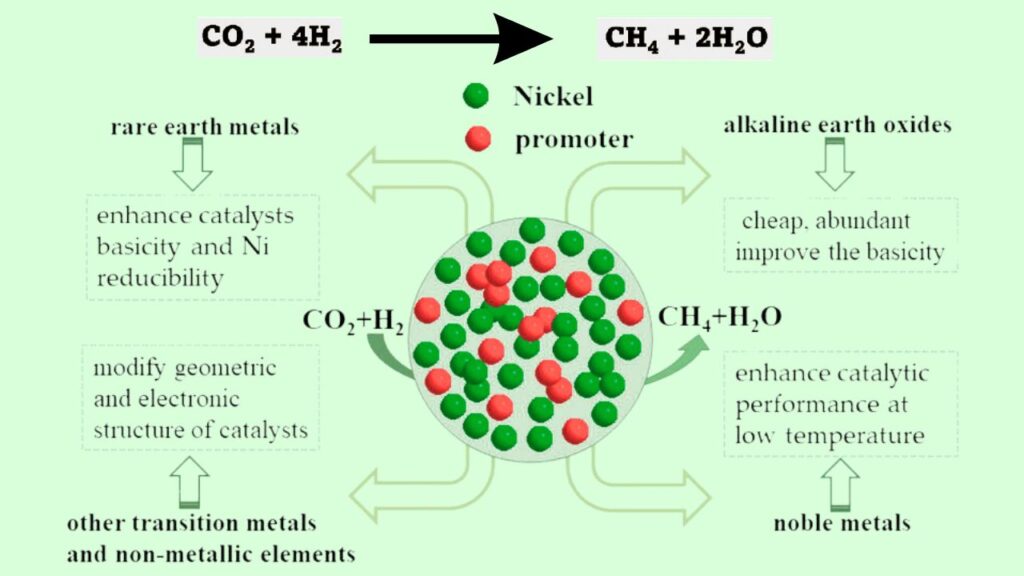
How Shape and Size Affect Catalytic Activity
The latest research highlights that the shape and size of nickel nanoparticles significantly impact their catalytic performance. Smaller or uniquely shaped particles can expose different atomic arrangements on their surfaces, which changes how they interact with CO₂ and hydrogen molecules.
Key Points:
- Smaller nanoparticles tend to have higher surface areas, increasing active sites.
- Certain geometrical shapes (like cubes or octahedrons) expose specific crystal facets that are more reactive.
- These structural factors enhance methane production rates and reduce unwanted by-products.
How Scientists Studied This
Researchers at Politecnico di Milano combined atomistic simulations with experimental validation to observe how changing nanoparticle shapes affected reaction rates. This approach enabled them to predict optimal particle morphologies before testing them in the lab, saving time and resources.
Practical Implications and Industrial Applications
Advancing Green Energy Technologies
The ability to design catalysts at the nanoscale means industries can develop more efficient processes to recycle CO₂. This is especially important for:
- Power-to-gas systems, where excess renewable energy produces hydrogen that reacts with CO₂ to generate methane.
- Synthetic natural gas (SNG) production, providing a renewable alternative to fossil natural gas.
- Chemical manufacturing, including ammonia synthesis and the Fischer–Tropsch process.
Economic and Environmental Benefits
Efficient CO₂ methanation catalysts can reduce operational costs by:
- Increasing reaction rates.
- Lowering energy requirements.
- Extending catalyst lifespans.
Environmentally, this technology supports:
- Carbon capture and utilization (CCU) strategies.
- Reduction of greenhouse gas emissions.
- Circular economy models.
How to Apply This Knowledge: A Step-by-Step Guide
If you’re working in catalysis research or industrial process design, here’s how to leverage the nickel nanoparticle insights:
Step 1: Select Target Reaction Conditions
Understand your operating temperature, pressure, and feedstock composition for the Sabatier reaction.
Step 2: Design Nickel Nanoparticles
- Use nanoparticle synthesis techniques (e.g., chemical vapor deposition, wet chemical methods).
- Focus on controlling particle shape and size based on research findings.
- Aim for shapes that expose the most active crystal facets.
Step 3: Characterize Nanoparticles
- Employ electron microscopy (TEM, SEM) to analyze shape and size.
- Use X-ray diffraction (XRD) for crystal structure confirmation.
Step 4: Test Catalytic Performance
- Conduct reactor experiments measuring methane yield and selectivity.
- Adjust synthesis parameters as needed to optimize activity.
Step 5: Scale Up
- Work with process engineers to integrate optimized catalysts into industrial setups.
- Monitor catalyst longevity and regeneration methods.
Bigger Molecules Now Proven to Extend Quantum Charge Flow Like Never Before
Universal Quantum Law Found In Superfluid Helium Shocks Physicists Worldwide
Electric Field Trick Revealed That Can Instantly Switch Off Superconductivity
FAQs About CO₂ to Methane Conversion
What is the Sabatier reaction?
The Sabatier reaction converts CO₂ and hydrogen into methane and water, using a catalyst like nickel. It’s important for producing renewable methane fuel.
Why is nickel preferred over other metals?
Nickel is less expensive and more abundant than precious metals but still offers good catalytic activity, making it practical for industrial applications.
How does nanoparticle shape influence catalysis?
Different shapes expose different atomic surfaces or facets, which interact with reactant molecules differently, affecting reaction rates and product selectivity.
Can this technology reduce carbon emissions significantly?
Yes. By recycling CO₂ into usable fuels, this method can lower net greenhouse gas emissions and support carbon-neutral energy systems.