Understanding the tiny charges inside atoms and molecules has always fascinated scientists. Now, electron diffraction is helping chemists uncover these partial atomic charges with unprecedented clarity. This exciting breakthrough is creating a buzz in the scientific community because it bridges a gap between theory and experiment, offering new insights into chemistry, materials science, and pharmaceuticals. But what exactly are partial atomic charges, and how does electron diffraction reveal them? Let’s dive in and explore this fascinating phenomenon in a simple yet expert way.
Table of Contents
What Are Partial Atomic Charges?
Imagine a molecule as a tiny team of atoms holding hands through chemical bonds. Sometimes, the electrons in these bonds don’t stay exactly between the atoms but lean more toward one atom than the other. This uneven sharing causes parts of atoms to have a tiny positive or negative charge. These tiny charges are called partial atomic charges or net atomic charges, and they are not whole numbers like the charges of ions but fractions.
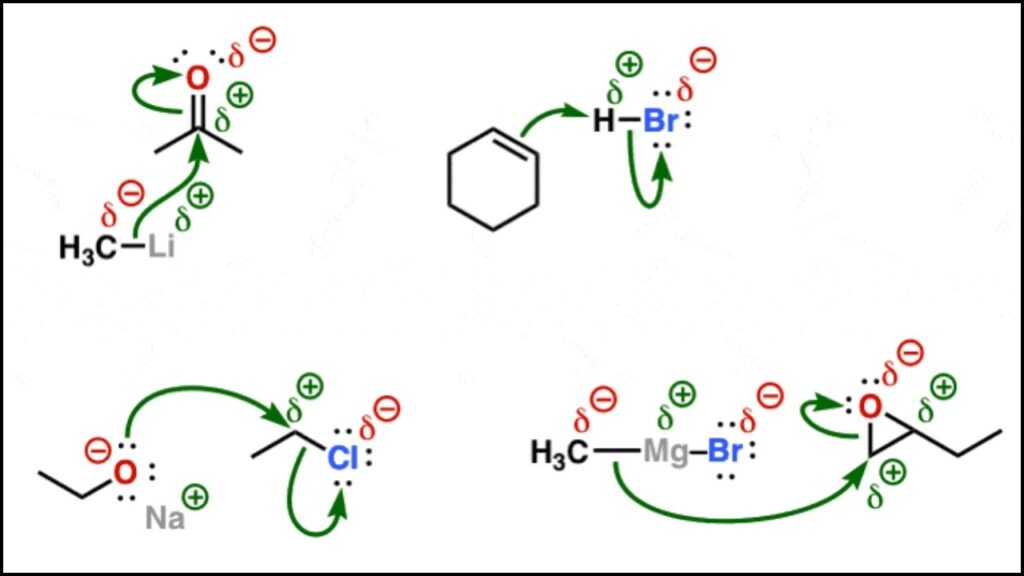
For example, in a hydrogen chloride (HCl) molecule, chlorine pulls electrons closer than hydrogen, giving chlorine a small negative charge and hydrogen a small positive charge. This charge difference helps explain why some molecules behave the way they do in reactions, bonding, and interactions.
How Does Electron Diffraction Help?
Electron diffraction uses beams of electrons to explore the innermost details of materials. When electrons pass through or bounce off a sample, they scatter due to electromagnetic forces of the atoms, creating a unique diffraction pattern. This pattern carries rich information about the atomic arrangement and—including recent advances—can disclose how electrons are distributed around atoms. This allows scientists to directly measure the partial charges on each atom.
Unlike traditional X-ray diffraction, electron diffraction is more sensitive to charge differences because electrons themselves are charged particles influenced strongly by electrical forces. Thanks to new techniques incorporating crystal structure refinement and ionic scattering factors, researchers can analyze the diffraction data to deduce how charges are distributed in molecules, even detecting tiny variations around hydrogen atoms.
Electron Diffraction Unlocks Partial Atomic Charges
| Feature | Details |
|---|---|
| What it measures | Partial atomic charges in molecules |
| Principle | Scattering of electron beams affected by atomic charges |
| Sensitivity | High enough to detect subtle charge differences and hydrogen atoms |
| Applications | Pharmaceuticals, materials science, chemistry research |
| Sample size requirement | Nanogram amounts of substances |
| Technique novelty | Combines electron diffraction with crystal refinement and ionic scattering |
| Recent breakthrough publication | August 2025, Nature article on experimental determination of partial charges |
| Official reference for partial charges methods | Materials Project: Methods for atomic partial charges |
The ability to experimentally measure partial atomic charges using electron diffraction is a landmark advance in chemistry and materials science. It offers direct, precise, and reliable insights into how electrons distribute within molecules—knowledge that’s vital for chemical understanding and innovation. This new approach not only empowers researchers to deepen their understanding but also revolutionizes practical applications like drug design and materials engineering.
As this technique becomes more widespread, it will transform how scientists explore the invisible world of atomic charges—turning theory into real-world data and expanding what’s possible in science and technology.
The Science Behind Partial Charges and Electron Diffraction
What Is a Partial Charge?
A partial charge is the fractional electrical charge an atom carries within a molecule due to the uneven sharing of electrons in a bond. It is usually represented by the Greek letter delta (𝛿) with a plus or minus to indicate positive or negative partial charge, e.g., 𝛿+ or 𝛿−.
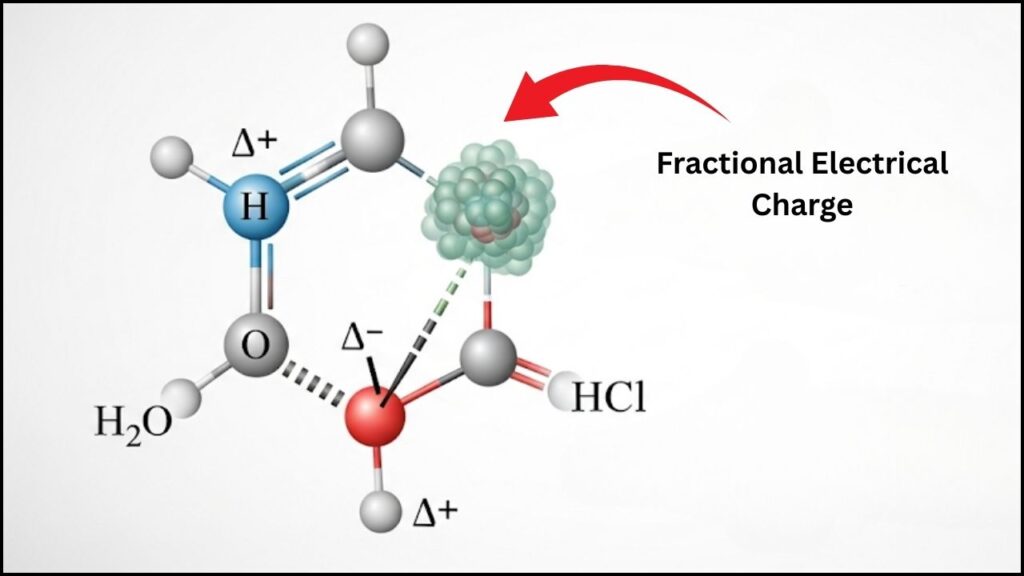
These charges arise because atoms have different electronegativities—a measure of how strongly they attract electrons. When atoms in a bond differ, electrons spend more time closer to the more electronegative atom, causing partial charges.
The Role of Electron Diffraction
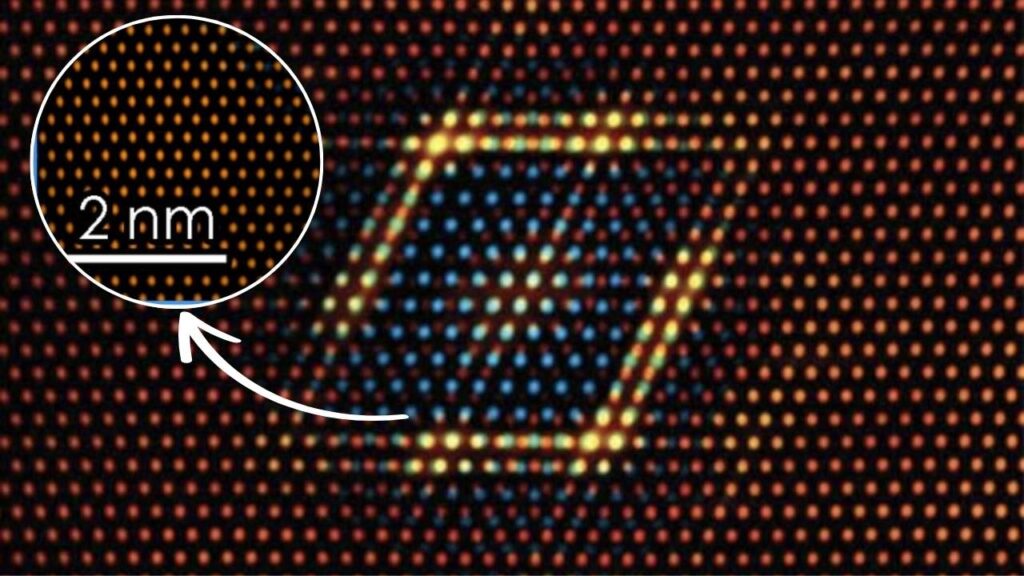
Electron diffraction involves shining a focused beam of electrons onto a very thin sample—often a nanocrystal just a few hundred atoms thick. The electrons scatter off the atoms, creating a diffraction pattern that reflects how electrons and nuclei are arranged.
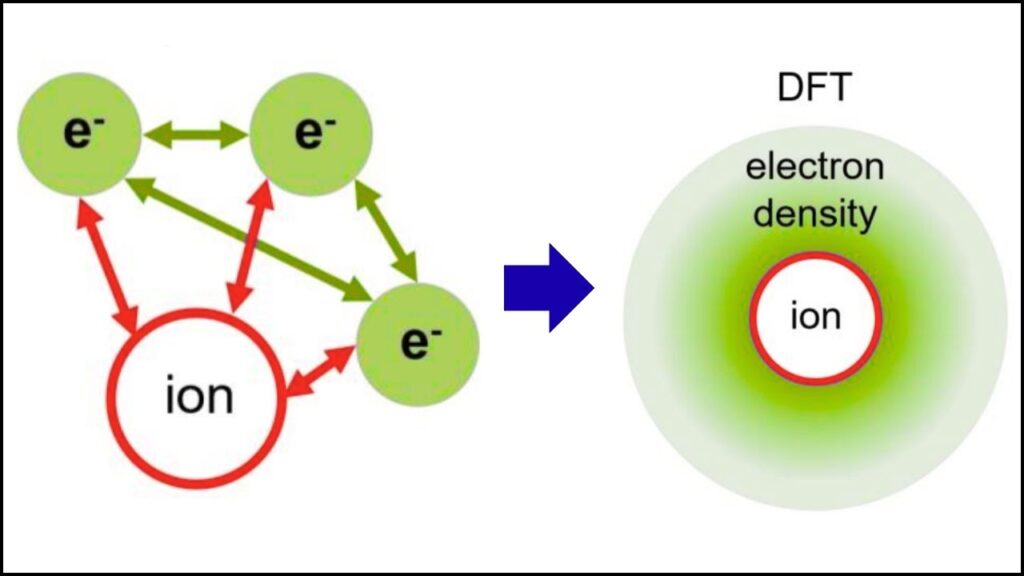
Because electrons feel the electrical fields of the atoms and electrons inside the material, their diffraction pattern reveals not just where atoms are, but also how electrons are distributed around them. By carefully analyzing the pattern, scientists can extract charge density maps, which show where electrons concentrate and where there’s a deficiency, leading to the discovery of partial atomic charges.
Recent Advances in Technique
The classic challenge was separating the effect of atomic positions from electron distribution in diffraction data. Researchers from the University of Vienna and others have developed a novel approach utilizing electron diffraction combined with crystal structure refinement and ionic scattering factor models. This approach is integrated into standard electron crystallography workflows and does not require specialized software.
The breakthroughs include:
- High precision: Ability to detect partial charges on all atoms, including hydrogens.
- Small sample size: Works with tiny amounts of material (nanograms).
- Reduced noise: Improves accuracy of molecular models by filtering data noise.
- Broad applicability: From amino acids to complex pharmaceutical molecules.
Why Are Partial Charges Important?
Partial atomic charges are fundamental to understanding the chemistry of molecules:
- Chemical Reactivity: They help predict how molecules interact and react. For example, regions of positive or negative charge guide how molecules bind to receptors or catalysts.
- Molecular Modeling: Simulating materials and biological molecules depends on accurate charge assignments.
- Drug Design: Pharmaceuticals’ effectiveness is often determined by charge-based interactions with biological targets.
- Material Science: Charge distribution affects electronic properties, like conductivity.
For decades, estimating partial charges depended heavily on computational approximations using theoretical methods like Density Functional Theory (DFT) or population analysis. While useful, these methods can vary widely and sometimes lack experimental confirmation. Electron diffraction now provides direct experimental validation.
Step-by-Step Guide: How Electron Diffraction Unlocks Partial Atomic Charges
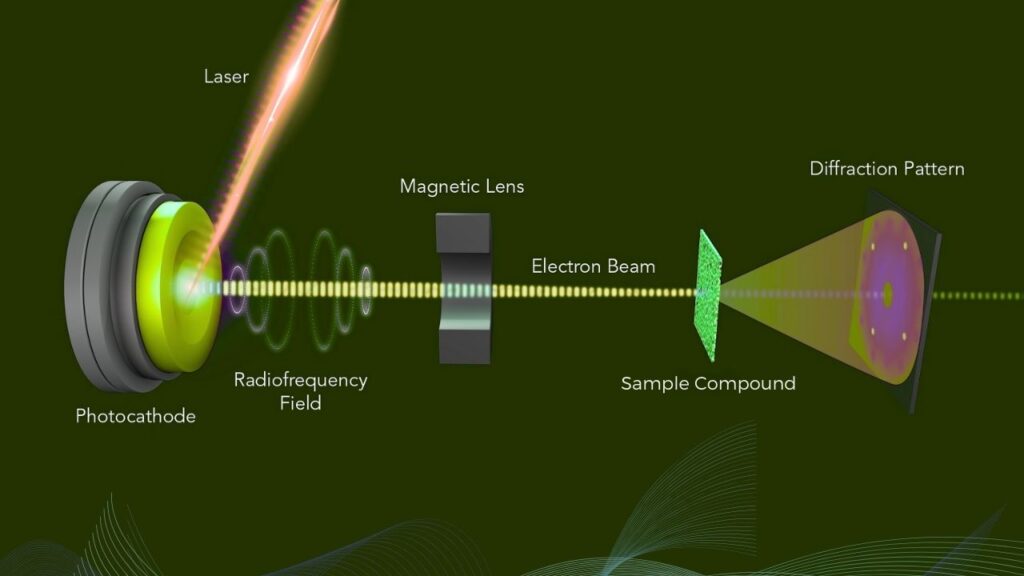
Step 1: Sample Preparation
- Obtain a high-quality crystal sample—usually nanometer thin sheets of the molecule of interest.
- Mount the sample onto the electron microscope stage for analysis.
Step 2: Electron Diffraction Experiment
- Direct a focused, coherent electron beam through the thin sample.
- Electrons scatter elastically from the atoms and their electron clouds.
- Diffraction patterns of electron intensities are recorded in a detector.
Step 3: Data Analysis and Refinement
- Use algorithms to refine the crystal structure from diffraction patterns.
- Incorporate ionic scattering factors to account for how charges alter electron scattering.
- Generate detailed electron density maps, showing charge distribution.
Step 4: Extract Partial Charges
- Analyze refined data to quantify partial atomic charges on each atom.
- Compare and validate using known standards or complementary theoretical methods.
Step 5: Apply Insights
- Use the charge data for better molecular modeling.
- Guide chemical synthesis, materials design, or drug development.
- Publish and share data for wider scientific community use.
Practical Applications of This Breakthrough
- Pharmaceuticals: Design drugs with improved binding based on charge distributions.
- Materials Science: Develop new materials with tailored electronic properties.
- Environmental Chemistry: Study pollutants and catalysts with accurate charge insights.
- Education and Research: Provide a new tool for chemists to deeply understand molecules beyond traditional methods.
Record-Breaking “Watermelon” Nucleus Discovered — Can It Rewrite Atomic Theory?
AI Tool Reveals Atomic Secrets of Ultra-Thin Films — A New Era in Materials Science
MicroscopyGPT Uses AI to Describe Atomic Structures in 2D Materials with Unmatched Precision
FAQs About Electron Diffraction Unlocks Partial Atomic Charges
1. What is the difference between electron diffraction and X-ray diffraction?
While both techniques analyze atomic structures, electron diffraction uses electrons, which are charged particles, and is more sensitive to charge distributions and small samples. X-ray diffraction uses X-rays, which interact primarily with electron clouds but are less sensitive to subtle charge differences.
2. Can electron diffraction detect hydrogen atoms?
Yes. Unlike many diffraction techniques where hydrogen is hard to see, electron diffraction’s sensitivity and new analysis methods allow detection of hydrogen atoms and their partial charges.
3. Why are partial atomic charges important for chemists?
Partial charges explain the behavior of molecules in chemical reactions, interactions, and properties. They are key to predicting molecule behavior, drug binding, and material characteristics.
4. How reliable are these electron diffraction charge measurements?
Recent studies published as recently as August 2025 demonstrate that these measurements are precise, reproducible, and align well with theoretical predictions, thus offering reliable experimental data.
5. What software or equipment is needed?
No specialized software beyond standard electron crystallography tools is required, making this technique accessible within modern electron microscopy labs.