In recent years, the engineered Cu-SSZ-13 catalyst has emerged as a groundbreaking solution for reducing harmful emissions of nitrogen oxides (NOx) and ammonia (NH3) from diesel engines. This catalyst plays a crucial role in Selective Catalytic Reduction (SCR) technology, which cleans exhaust gases by converting harmful pollutants into harmless nitrogen and water. Understanding how this catalyst works and why it is winning praise in clean chemistry is vital for embracing greener industrial and automotive solutions.
This article will guide you through the science behind the Cu-SSZ-13 catalyst, its advantages, and how it transforms emission control. We will also explore the reasons this catalyst is becoming an industry standard, backed by clear data and recent research insights.
Table of Contents
Engineered Cu-SSZ-13 Catalyst Cuts Harmful NH3 Emissions
| Feature | Details |
|---|---|
| Target Pollutants | Nitrogen oxides (NOx), Ammonia (NH3) |
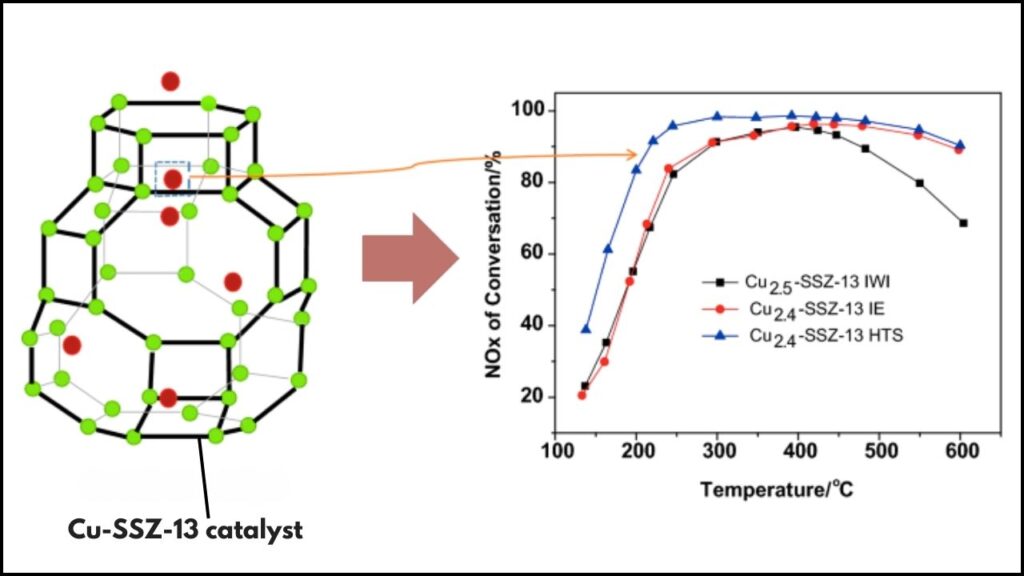
| Catalyst Name | Cu-SSZ-13 (Copper-exchanged SSZ-13 zeolite) |
| Reaction Temperature Range | 150 to 550 °C |
| Key Reaction | Selective Catalytic Reduction (SCR): NOx + NH3 → N2 + H2O |
| Advantages | Wide temperature activity window, high hydrothermal stability, and resistance to poisoning |
| Active Sites | Copper ions (Cu2+) located in zeolite framework |
| Industrial Impact | Significant cut in vehicle emissions, enhanced air quality, compliance with emission standards |
| Practical Use | Diesel engine exhaust after-treatment |
| Official Website | Cu-SSZ Catalyst Research |
The engineered Cu-SSZ-13 catalyst is a revolutionary advancement in clean chemistry and emission control. By enabling efficient selective catalytic reduction of harmful nitrogen oxides and ammonia in diesel exhaust, it significantly improves air quality and supports environmental health. Built from a unique copper-exchanged zeolite structure, this catalyst combines wide temperature activity, durability, and high pollutant conversion rates in real-world conditions.
Understanding its working mechanism and optimization strategies provides industries and environmental agencies with a powerful tool for greener technologies. As diesel engines remain a major source of NOx emissions worldwide, Cu-SSZ-13 stands as a critical piece in the global effort toward cleaner air and sustainable transportation.
What is Cu-SSZ-13 and Why Does it Matter?
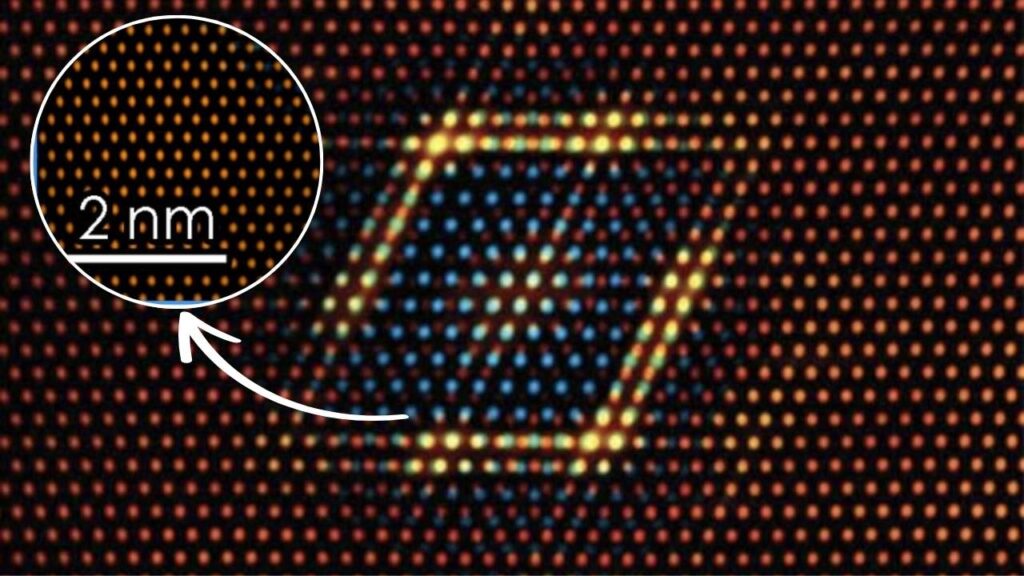
Cu-SSZ-13 is a copper-exchanged zeolite catalyst designed for the selective catalytic reduction of NOx emissions in diesel exhaust systems. Zeolites are microporous mineral frameworks with unique properties that allow them to support catalytic metals such as copper. SSZ-13 is a type of small-pore zeolite that holds copper ions in specific locations, enabling efficient reactions.
Nitrogen oxides, primarily NO and NO2, are harmful pollutants produced by burning fossil fuels. These gases contribute to smog, acid rain, and respiratory problems. Controlling NOx emissions is critical to improving air quality and public health.
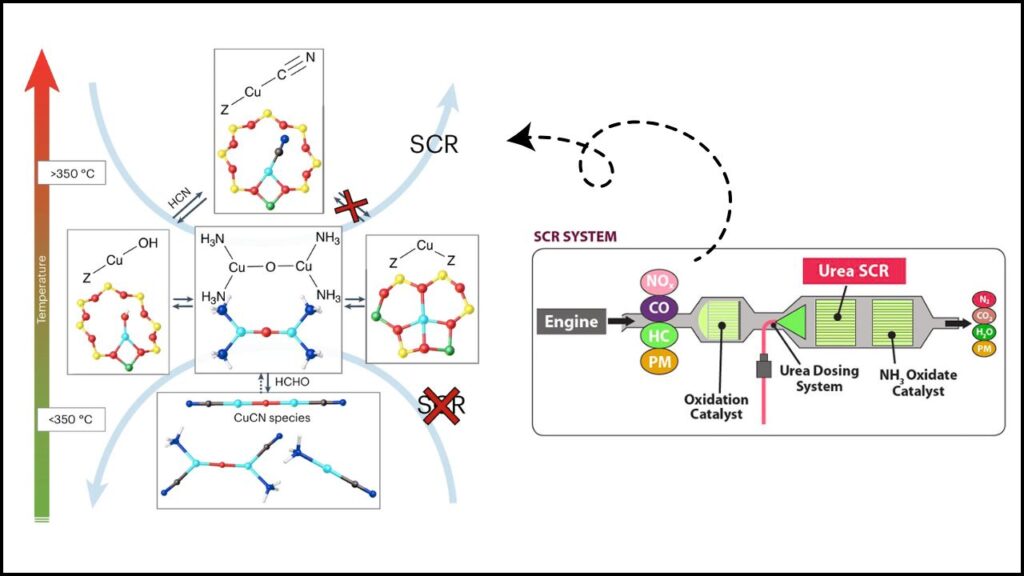
The Cu-SSZ-13 catalyst facilitates the SCR reaction, where NOx is reduced by ammonia (NH3) to produce environmentally benign nitrogen (N2) and water (H2O). This reaction typically occurs in diesel vehicle exhaust systems to meet stringent emission standards.
How Does the Cu-SSZ-13 Catalyst Work?
At the heart of the SCR technology is the catalysis of a reaction that converts harmful NOx to neutral gases. The copper ions inside the SSZ-13 framework serve as active sites for this reaction. Here’s a simplified breakdown of what happens:
1. Absorption of Reactants
NOx gases and ammonia diffuse into the catalyst’s micropores and interact with copper active sites.
2. Catalytic Cycle
- Reduction half-cycle: NOx molecules (NO and NO2) react with copper ions and ammonia to form nitrogen gas through intermediate compounds, such as nitrosamines.
- Oxidation half-cycle: Copper changes its oxidation state between Cu(I) and Cu(II) throughout the reaction, with oxygen playing a key role in reactivating copper sites to continue the catalysis.
3. Release of Harmless Gases
The reaction releases N2 (nitrogen gas) and H2O (water vapor), which exit the exhaust system safely.
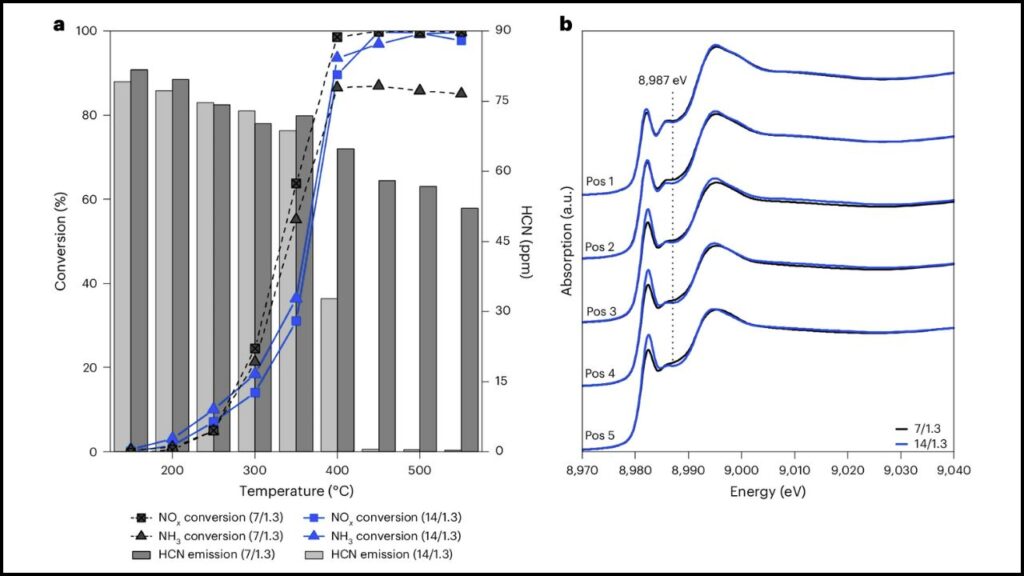
Studies based on density functional theory (DFT) calculations have revealed the detailed mechanism, including coordination geometries of copper ions and intermediate species formed during reactions on Cu-SSZ-13. The catalyst’s structure helps stabilize reaction intermediates and promotes efficient cycling between copper oxidation states, resulting in high NOx conversion efficiency even at low to mid exhaust temperatures (150–550°C).
Why is Cu-SSZ-13 Superior to Other Catalysts?
The Cu-SSZ-13 catalyst stands out because of its:
- Wide Active Temperature Range: It works effectively between 150°C and 550°C, covering the range typical of diesel exhaust systems.
- Hydrothermal Stability: This catalyst maintains performance even in the presence of water vapor and high temperatures, which degrade many other catalysts.
- Resistance to Contaminants: It resists aging and poisoning from sulfur, hydrocarbons, and alkali metals often found in exhaust gases.
- High NOx Conversion Efficiency: Converts up to 90-95% of NOx under optimal conditions.
- Maintains High Activity at Large Space Velocities: Handles high flow rates of exhaust gases without losing efficiency.
These attributes allow Cu-SSZ-13 to meet tough emission regulations worldwide efficiently and reliably.
Practical Implications for Industry and Environment
Cu-SSZ-13’s application in diesel engine after-treatment systems has a direct environmental impact:
- Reduction in Airborne NOx: Lower concentration of NOx reduces smog formation and respiratory diseases.
- Lower Ammonia Slip: Engineered Cu-SSZ-13 effectively controls ammonia emissions, preventing secondary pollution.
- Improved Vehicle Compliance: Helps automakers meet evolving emission standards globally.
- Sustainable Emission Control: The catalyst’s durability extends the lifetime of emission control systems, reducing overall costs and waste.
By integrating Cu-SSZ-13 catalytic converters, cities can see cleaner air and fewer health-related issues caused by vehicle emissions, promoting a healthier environment.
How to Optimize Cu-SSZ-13 Performance
Research highlights that the catalyst’s performance depends on factors including copper content and distribution, zeolite morphology, and co-doping with other metals:
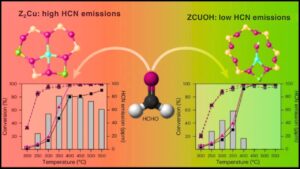
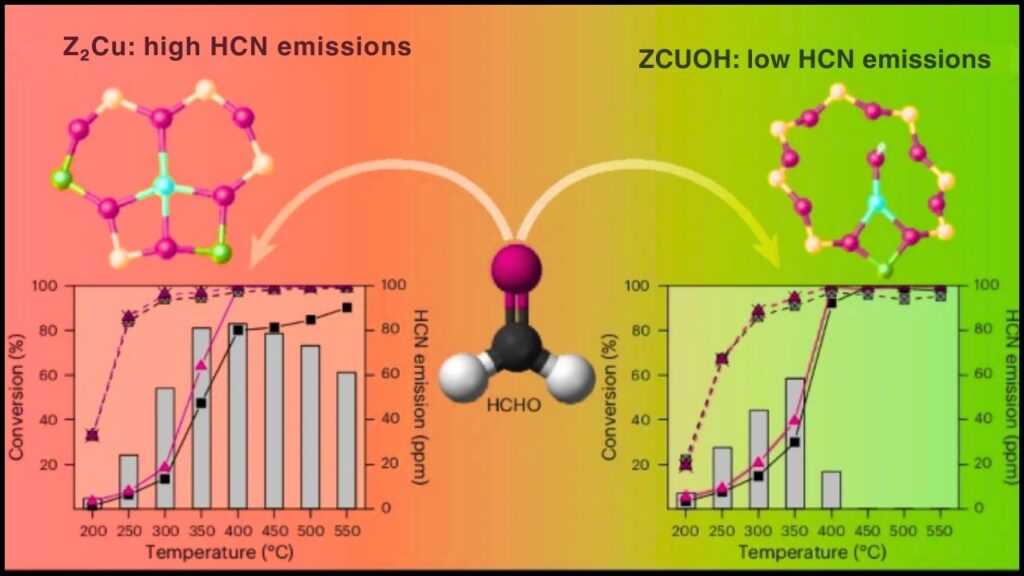
- Copper Content and Cu/Al Ratio: Adjusting copper loading optimizes the number and type of active copper sites. Higher Cu/Al ratios improve low-temperature activity but must balance stability.
- Site Engineering: Increasing the proportion of certain copper species (like ZCuOH) enhances low-temperature activity, while others (like Z2Cu) contribute to hydrothermal stability.
- Introducing Heteroatoms: Additions of metals like iron (Fe) can improve activity at different temperature ranges by complementing copper’s function.
- Morphology Control: Controlling crystal size and distribution can enhance catalyst exposure to exhaust gases and improve overall efficiency.
These strategies have been actively studied and implemented to extend the catalyst lifetime and boost performance, making Cu-SSZ-13 highly adaptable for various emission control scenarios.
New Carbon Catalyst Could Revolutionize Biodiesel — No Metals Needed
Engineered Ruthenium Catalysts: A Sustainable Revolution for Hydrogen and Ammonia
New Triple-Layer Catalyst Boosts Green Hydrogen Output by 800%—A Game-Changer for Clean Energy
FAQs About Engineered Cu-SSZ-13 Catalyst Cuts Harmful NH3 Emissions
Q1: What pollutants does Cu-SSZ-13 target?
Cu-SSZ-13 primarily targets nitrogen oxides (NO and NO2) and ammonia (NH3) in diesel exhaust. It reduces NOx to nitrogen gas and water, significantly cutting harmful emissions.
Q2: How does the catalyst handle high temperatures and water vapor?
Cu-SSZ-13 is engineered to withstand hydrothermal aging, maintaining its structure and activity even under moist, high-temperature exhaust conditions.
Q3: Is Cu-SSZ-13 used in regular cars?
Yes, many modern diesel vehicles use catalysts based on Cu-SSZ-13 in their exhaust after-treatment systems to meet strict emission standards.
Q4: What makes copper catalyst sites special?
Copper in Cu-SSZ-13 exists in distinct sites within the zeolite framework, each with unique reactivity and stability, enabling efficient SCR reactions across various temperatures.
Q5: Can Cu-SSZ-13 reduce ammonia slip?
Yes, it effectively reduces both NOx and controls ammonia slip, preventing secondary pollution from excess NH3 emissions.