Fluorescent Molecules: Fluorescent molecules are at the heart of some of the most exciting discoveries in science today. They help us see the invisible—lighting up the inner workings of living cells, tracing the history of rocks beneath our feet, and even revealing the chemistry of distant galaxies. But perhaps the most fascinating story is how these tiny glowing molecules can withstand extreme environments using new techniques. From the icy darkness of deep caves to the radiation-filled void of interstellar space, fluorescent molecules are proving to be far more resilient than anyone imagined.

In this article, we’ll explore how scientists are uncovering the secrets of these remarkable molecules, what new techniques are making them even tougher, and how this knowledge could shape the future of fields as diverse as astrobiology, medicine, and environmental science. Whether you’re a student, a professional, or just a curious mind, you’ll find valuable insights and practical examples that make this topic both approachable and authoritative.
Fluorescent Molecules
| Key Point | Details |
|---|---|
| Survival in Space | Polycyclic aromatic hydrocarbons (PAHs) use recurrent fluorescence to survive intense UV and cosmic rays in interstellar clouds. |
| Cave Chemistry & Life | Fluorescent minerals in caves help scientists understand how life persists in dark, mineral-rich environments, with implications for extraterrestrial life. |
| Non-Destructive Analysis | Handheld spectrometers allow non-invasive mapping of fluorescent cave minerals, building a public chemical fingerprint database. |
| Redox-Switchable Dyes | New fluorescent dyes can switch emission color in response to redox changes, enabling deep-tissue imaging and environmental sensing. |
| Extreme Stability Nanoparticles | Dual-emission carbon nanoparticles maintain performance under extreme pH and temperature, ideal for harsh environments. |
| Official Resource | For more, visit the James Webb Space Telescope official site. |
Fluorescent molecules are more than just scientific curiosities—they are powerful tools that illuminate the secrets of the universe, from the tiniest cell to the vastness of interstellar space. Thanks to new techniques like recurrent fluorescence, redox-switchable dyes, and dual-emission nanoparticles, these molecules can now withstand and thrive in the harshest environments known to science. Their resilience not only advances our understanding of life’s potential beyond Earth but also drives innovation in medicine, environmental science, and industry. As we continue to explore the extremes, fluorescent molecules will light the way—quite literally—into the unknown.
What Are Fluorescent Molecules?
Fluorescent molecules are compounds that absorb light at one wavelength and emit it at another, longer wavelength. This property—fluorescence—makes them glow when exposed to specific types of light, such as ultraviolet (UV).
Scientists use this glow to study everything from the structure of proteins to the composition of distant cosmic clouds.
Why Are They Important?
- Biology: Track proteins, visualize living cells, and diagnose diseases.
- Geology: Reveal the chemical history of rocks and minerals.
- Astronomy: Detect organic molecules in space, helping us understand the origins of life.
However, these molecules often face hostile environments—intense radiation, extreme temperatures, or reactive chemicals—that can break them apart or make them stop glowing. The quest to make fluorescent molecules tougher is at the cutting edge of modern science.
How Do Fluorescent Molecules Survive Extreme Environments?
1. Recurrent Fluorescence: The Space Survival Mechanism
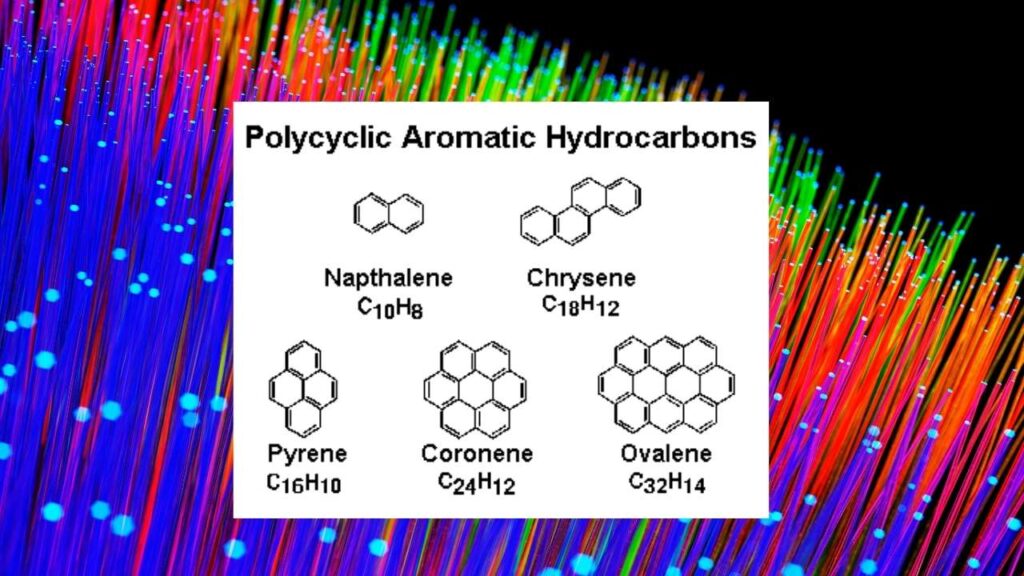
The James Webb Space Telescope (JWST) has provided a new window into the cosmos, revealing how polycyclic aromatic hydrocarbons (PAHs)—large, flat organic molecules—survive in the harshest interstellar environments. These molecules are bombarded by high-energy ultraviolet radiation and cosmic rays in cold molecular clouds, conditions that should destroy them. Yet, PAHs are found everywhere in space, holding up to 10–25% of the galaxy’s carbon—an essential element for life.
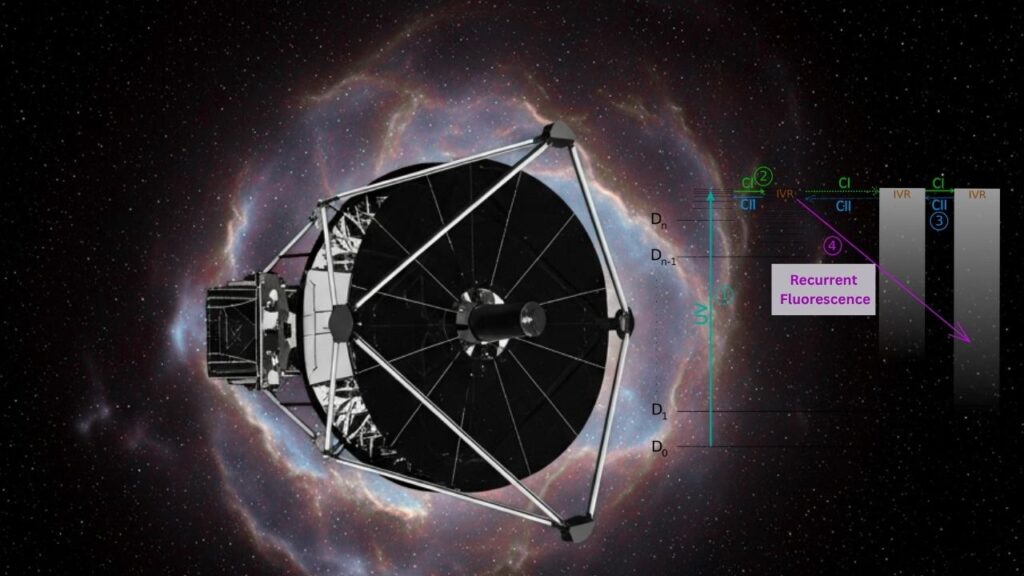
How Do They Survive?
A recent study published in Physical Review Letters found that closed-shell PAHs, such as the indenyl cation, use a process called recurrent fluorescence to dissipate excess energy. Instead of breaking apart, these molecules absorb energy and quickly release it as light, before it can cause damage. This mechanism, combined with infrared emission, allows PAHs to resist fragmentation and ionization, making them far more robust than previously believed.
Example:
Think of a PAH as a trampoline. When hit by a cosmic ray, it bounces (absorbs energy). Instead of breaking, it flashes with light (releases energy) and lands safely, ready for the next hit.
Why It Matters:
- PAHs serve as a major carbon reservoir in the galaxy.
- Their resilience hints at how organic molecules could survive the journey from space to planets, possibly seeding life.
2. Fluorescent Caves: Earth’s Model for Extraterrestrial Life
Deep underground, caves like South Dakota’s Wind Cave and Minnesota’s Mystery Cave reveal a hidden world of glowing minerals. Under UV light, these minerals shine in vibrant pinks, blues, and greens.

Scientists, led by astrobiologist Joshua Sebree, are using these caves as natural laboratories to understand how life survives in dark, mineral-rich, and isolated environments.
What Do They Find?
- Chemical Fossils: Fluorescent minerals mark ancient water flows and chemical changes, offering a timeline of the cave’s formation.
- Non-Destructive Mapping: Using handheld spectrometers, researchers collect fluorescence spectra—chemical fingerprints—without damaging the cave.
- Database Building: Students are creating a public database of these fingerprints, adding a new dimension to cave mapping.
- Astrobiology Models: The chemistry of these caves is similar to what might be found on icy moons like Europa, making them ideal models for studying how life could persist in extraterrestrial environments.
Challenges:
Fieldwork is tough—crawling through tight tunnels, standing in freezing water, and keeping instruments warm in 9°C (48°F) caves. Yet, the rewards are significant: new insights into cave formation, mineral chemistry, and the potential for life on other worlds.
3. Redox-Switchable Dyes: Tunable Fluorescence for Harsh Conditions
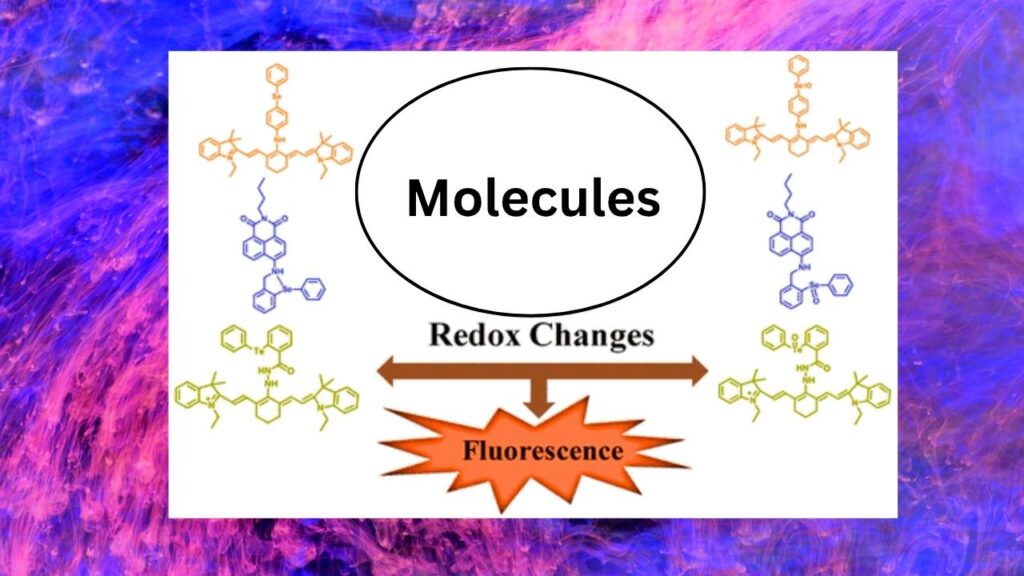
Fluorescent dyes that can change their emission color in response to redox changes are opening new possibilities for imaging and sensing in extreme environments. Researchers at the Shibaura Institute of Technology have developed nitrogen-rich pyrazinacene dyes that can switch between visible and near-infrared (NIR) emission by altering their redox state.
Why Is This Important?
- Deep Tissue Imaging: NIR-emitting dyes can penetrate deeper into biological tissues, making them valuable for medical diagnostics.
- Environmental Sensing: Redox-responsive dyes can detect changes in chemical environments, such as those found in polluted soils or extreme industrial settings.
- Versatility: The new design strategy—intramolecular charge transfer—enables compact molecules to exhibit dramatic shifts in fluorescence, enhancing their utility.
Data:
- Octaazatetracene emits at 560 nm (yellow) when reduced and at 847 nm (NIR) when oxidized, with a quantum yield of 16.4% in the NIR state—comparable to leading NIR dyes.
4. Extreme Stability Nanoparticles: Dual-Emission for Sensing
Carbon-based nanoparticles with dual-emission properties are another breakthrough in the field. These DE-CNPs (dual-emission carbon nanoparticles) are stable under extreme pH and temperature conditions, maintaining their sensing performance even in harsh environments.
Applications:
- Environmental Monitoring: Detecting toxins or changes in water chemistry in polluted or remote locations.
- Medical Diagnostics: Stable fluorescence allows for reliable imaging in variable physiological conditions.
- Industrial Processes: Monitoring chemical reactions or leaks in high-temperature or corrosive environments.
5. Practical Field Techniques: Non-Destructive Data Collection
One of the most valuable advances is the ability to study fluorescent molecules and minerals in extreme environments without causing damage. Scientists use handheld spectrometers to collect fluorescence spectra directly from cave walls, avoiding the need to break off samples. This approach:
- Preserves fragile environments.
- Enables rapid, in-situ analysis.
- Builds comprehensive chemical databases for future research.
Example:
A plain brown cave wall, under UV light, reveals a bright layer of fluorescent mineral, indicating where water pooled thousands of years ago. This information helps reconstruct the cave’s environmental history and supports studies of how life could persist underground.
Step-by-Step Guide: How Scientists Study Fluorescent Molecules in Extreme Environments
Step 1: Selecting the Right Molecule or Material
- Space Studies: Choose robust molecules like PAHs that resist radiation and cold.
- Bioimaging: Use redox-switchable or super-photostable dyes for long-term observation.
- Environmental Sensing: Opt for dual-emission nanoparticles or specialized probes.
Step 2: Preparing for Harsh Conditions
- Lab Simulation: Replicate extreme environments—UV exposure, high/low temperatures, or variable pH.
- Fieldwork: Use portable, rugged instruments; keep sensitive equipment warm in cold caves.
Step 3: Data Collection
- Imaging: Use advanced telescopes (JWST) or microscopes for capturing fluorescence.
- Spectroscopy: Gather chemical fingerprints with handheld spectrometers in the field.
Step 4: Data Analysis and Interpretation
- Compare fluorescence data with traditional chemical or geological methods.
- Build and reference public databases for cross-comparison and validation.
Step 5: Application and Innovation
- Use findings to improve dye design, develop new sensors, or inform astrobiology missions.
- Translate cave discoveries to models for life on other planets.
Robots and Smart Glasses Learn to Recognize Individuals in Real Time
Violent Solar Eruption Launches Coronal Mass Ejection Triggering Rare G4‑Level Geomagnetic Storm
Zinciodine Battery Offers a New Alternative to Lithium-Ion Technology
FAQs About Fluorescent Molecules
What is recurrent fluorescence, and why is it important?
Recurrent fluorescence is a process where a molecule absorbs energy and releases it as light before the energy can cause damage. This allows molecules like PAHs to survive in the harsh conditions of space.
How do fluorescent caves help us understand extraterrestrial life?
The chemistry and mineralogy of fluorescent caves on Earth resemble environments found on icy moons like Europa. Studying these caves helps scientists model how life could survive in similar conditions elsewhere in the solar system.
What are redox-switchable dyes, and where are they used?
Redox-switchable dyes change their fluorescence color in response to redox conditions. They are used in deep-tissue imaging, environmental sensing, and industrial monitoring.
How do scientists collect fluorescence data in caves?
Researchers use handheld spectrometers to non-destructively gather fluorescence spectra from cave walls, building chemical fingerprint databases without damaging the environment.
Can these techniques be used outside of research?
Yes! Fluorescent molecules and nanoparticles are already used in medical diagnostics, environmental monitoring, and industrial safety systems.
Where can I learn more?
For the latest in space chemistry, visit the James Webb Space Telescope official site. For cave and mineral fluorescence, check resources from the American Chemical Society.