Physicist Resolves Long-Standing Thermodynamics Question and Refines Einstein’s Model: In a remarkable scientific breakthrough, a physicist has finally resolved a 120-year-old question in thermodynamics, fundamentally refining Einstein’s model and reshaping our understanding of the laws that govern the universe. This achievement directly connects the Nernst heat theorem—a core principle in low-temperature physics—to the well-established second law of thermodynamics, overturning decades of debate and Einstein’s own interpretation.

Let’s explore what this means, why it’s important, and how it could change the way we teach and understand the very basics of physical science.
Physicist Resolves Long-Standing Thermodynamics Question and Refines Einstein’s Model
| Topic | Details |
|---|---|
| Breakthrough | Nernst heat theorem proven to follow from the second law of thermodynamics, not a separate third law |
| Key Figure | Prof. José María Martín-Olalla, University of Seville |
| Historic Opponent | Albert Einstein, who argued the theorem was an independent law |
| Implication | Refines how entropy and absolute zero are understood and taught |
| Nobel Connection | Walther Nernst, Nobel Prize in Chemistry, 1920 |
| Official Source | University of Seville |
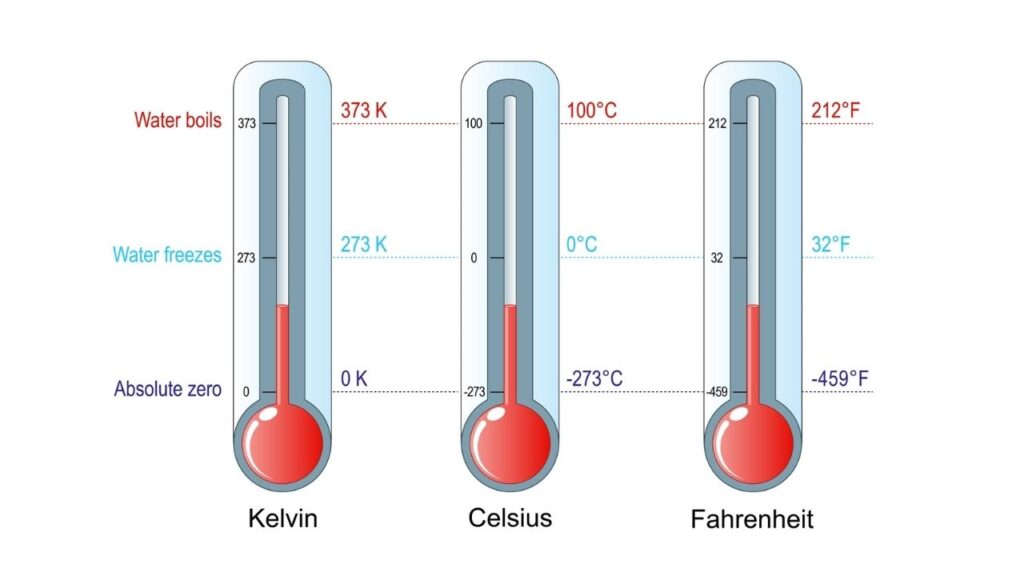
| Temperature Focus | Absolute zero: -273.15°C (0 Kelvin) |
| Career Impact | Could reshape the teaching of thermodynamics and the structure of its laws |
The recent resolution of the Nernst heat theorem debate marks a major milestone in physics, showing that the behavior of entropy at extremely low temperatures is a direct result of the second law of thermodynamics. This not only corrects a long-standing interpretation by Einstein but also unifies our understanding of the fundamental laws that govern the universe. The implications will ripple through science education, research, and technology for years to come.
What Is the Nernst Heat Theorem?
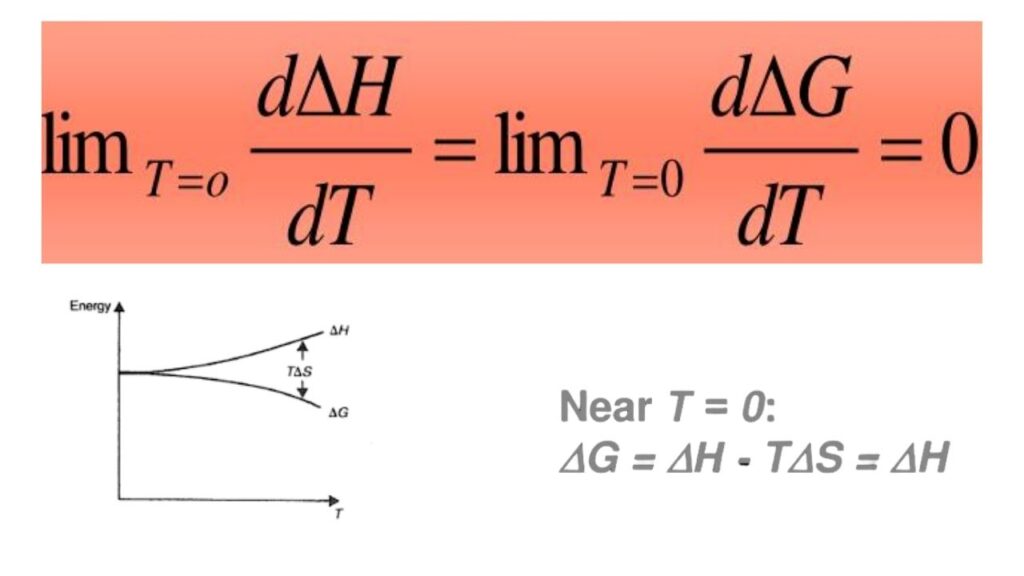
The Nernst heat theorem, proposed by Walther Nernst in 1905, states that as a system’s temperature approaches absolute zero (the coldest temperature possible), the entropy change (a measure of disorder or randomness) for any process also approaches zero. In simple terms: when things get extremely cold, there’s almost no “wiggle room” left for energy to spread out or change form.
Example for Kids and Pros
- For Kids: Imagine a box of marbles. At room temperature, the marbles can roll around and mix up easily. But as you make the box colder and colder, the marbles slow down and eventually stop moving. At absolute zero, they’re as still as possible—no more mixing or moving!
- For Professionals: The formal statement: As temperature (T) approaches zero, the change in entropy (ΔS) for any reversible process also approaches zero.
Why Was This Controversial?
Einstein vs. Nernst: The Great Debate
- Nernst’s View: Nernst argued that the theorem could be proven using thermodynamic arguments, and that it was a direct consequence of the second law of thermodynamics (which says entropy in the universe always increases or stays the same).
- Einstein’s Challenge: Albert Einstein disagreed. He believed that Nernst’s proof wasn’t rigorous enough and that the theorem should be treated as a separate, independent law—what we now call the “third law of thermodynamics.”
- The Core Issue: Could the behavior of entropy at extremely low temperatures be explained by existing laws, or did it require a new law entirely?
Why Does This Matter?
Understanding how entropy behaves near absolute zero is crucial for everything from designing super-efficient engines to developing quantum computers and understanding the fundamental limits of cooling materials.
The Breakthrough: A Modern Proof
Professor José María Martín-Olalla of the University of Seville has published a rigorous proof that settles the debate. His work shows that the Nernst heat theorem is not an independent law but can be mathematically derived from the second law of thermodynamics, using only thermodynamic arguments—no extra assumptions needed.
The Key Insight: The “Virtual Engine”
- What’s New? Martín-Olalla introduced the concept of a “virtual engine”—a hypothetical, perfectly efficient device that helps clarify the logical structure of the laws of thermodynamics.
- Why Is This Important? By considering what would happen if such an engine existed, he showed that the Nernst theorem naturally follows from the second law. There’s no need for a separate third law; the universe’s rules are more unified than previously thought.
Practical Implications
For Science and Engineering
- Unification of Laws: This proof narrows the scope of the third law, suggesting that the second law is sufficient to describe the behavior of entropy at low temperatures.
- Teaching and Textbooks: Expect future physics and chemistry textbooks to update how they present the laws of thermodynamics, possibly reducing the emphasis on the third law as an independent principle.
- Technology: Understanding the true limits of entropy at low temperatures helps in the design of ultra-cold systems for quantum computing, superconductors, and advanced refrigeration.
For Everyday Life
- Absolute Zero Is Still Unreachable: The proof reinforces that it is impossible to reach absolute zero in practice—no matter how good our technology gets, we can only get close, never all the way there.
- Why? Because reaching absolute zero would require an infinite number of steps or an infinite amount of time.
A Step-by-Step Guide: Understanding the Laws of Thermodynamics
1. First Law: Conservation of Energy
- Energy cannot be created or destroyed; it can only change forms.
2. Second Law: Entropy Always Increases
- The total disorder (entropy) of the universe always increases or stays the same. You can’t “unmix” things naturally.
3. Third Law (Now Refined): Entropy Approaches Zero at Absolute Zero
- As temperature approaches absolute zero, entropy change approaches zero. But this is now shown to be a consequence of the second law, not a separate law.
4. Absolute Zero: The Coldest Temperature
- Absolute zero (0 Kelvin or -273.15°C) is the theoretical point where all motion stops. It’s impossible to reach, but we can get very close.
Example: Cooling Down
If you try to cool a cup of water to absolute zero, you’ll find it gets harder and harder to remove the last bits of heat. No matter how long you try, you can never get all the way there.
The Historical Journey: From Nernst to Einstein to Today
- 1905: Walther Nernst formulates the heat theorem, based on experimental observations.
- 1911: Nernst presents his ideas at the First Solvay Congress, sparking debate.
- 1912: Nernst publishes a formal proof, arguing that absolute zero is unattainable.
- Einstein’s Objection: Einstein argues that the proof isn’t rigorous and that the theorem should stand alone as a third law.
- 2025: Martín-Olalla’s proof unifies the Nernst theorem with the second law, settling the debate.
Scientists Develop Breakthrough Copper Composite With Superior Strength and Conductivity
BTQ Launches Quantum-Safe Framework to Protect Stablecoins From Future Cyber Threats
FAQs About Physicist Resolves Long-Standing Thermodynamics Question and Refines Einstein’s Model
What is the Nernst heat theorem in simple terms?
It says that as things get colder and colder, the amount of disorder (entropy) that can change in a system gets smaller and smaller, and at absolute zero, it’s basically zero.
Why couldn’t Einstein and Nernst agree?
Nernst thought his theorem followed from existing laws, but Einstein argued it needed to be a new, separate law. They disagreed on whether the proof was rigorous enough.
What does this new proof mean for science?
It means we can explain the behavior of entropy at low temperatures using only the second law of thermodynamics. This simplifies our understanding of how the universe works.
Can we ever reach absolute zero?
No. The laws of physics say it would take forever (or infinite steps) to get there, so it’s impossible in practice.
Will this change how thermodynamics is taught?
Yes! Future textbooks and courses may combine or simplify the third law, focusing more on the second law as the foundation.