Green hydrogen—hydrogen produced using renewable electricity—is widely seen as a cornerstone of a cleaner, greener future. But up until now, a stubborn roadblock has slowed its progress: the high cost of production. A team of international scientists led by Monash University has overcome this hurdle by stabilizing cobalt-based catalysts, which could soon replace the costly and rare iridium needed in today’s most advanced hydrogen electrolyzers. Published in the prestigious journal Nature Energy, this breakthrough is a game-changer for sustainable energy, promising to accelerate global decarbonization and bring green hydrogen to the masses.
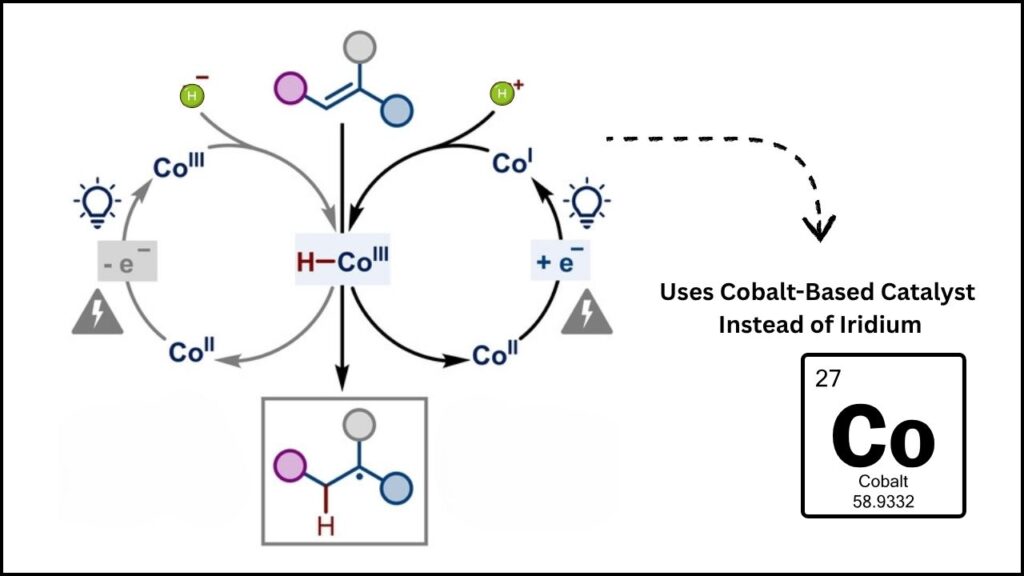
Imagine a world where trucks, ships, factories, and even homes could be powered by a fuel that produces only water as a byproduct—no greenhouse gases, no air pollution. That’s the promise of green hydrogen. But turning this vision into reality has been held back by the scarcity and expense of iridium, a metal so rare that it’s more valuable than gold and just as hard to find. The Monash-led team’s discovery not only solves this problem with cobalt—a far more abundant and affordable metal—but also gives engineers a clear blueprint for designing the next generation of electrolyzers. The result? Cheap, large-scale green hydrogen within reach, with global implications for climate change, industry, and jobs.
Table of Contents
Monash Breakthrough
| Topic | Key Insight | Why It Matters |
|---|---|---|
| Catalyst Material | Cobalt can replace iridium in green hydrogen electrolyzers | Cobalt is cheaper and far more abundant |
| Stability Breakthrough | Degradation and catalytic activity are independent in cobalt anodes | Allows separate optimization for each aspect |
| Scalability | Enables large-scale, affordable green hydrogen production | Removes iridium supply bottleneck |
| Research Methods | Advanced spectroscopic, electrochemical, and computational techniques | Ensures findings are robust and reproducible |
| Global Impact | Supports decarbonization of energy and chemical industries worldwide | Green hydrogen can now scale globally |
The Monash University-led breakthrough is a milestone in the fight against climate change. By stabilizing cobalt-based catalysts, the team has opened the door to affordable, large-scale green hydrogen production—without reliance on rare, costly metals. This achievement isn’t just about hydrogen. It’s about cleaner air, new jobs, and a more secure energy future for everyone, everywhere.
Why Green Hydrogen Matters
Green hydrogen is different from the hydrogen most commonly made today, which comes from fossil fuels and releases carbon dioxide. In contrast, green hydrogen uses renewable electricity—from solar panels, wind turbines, or hydroelectric plants—to split water molecules into hydrogen and oxygen. The only thing that comes out is water vapor, making it truly clean energy.
But what’s the catch? Right now, green hydrogen is costly, mainly because of the catalyst—a chemical helper—that’s needed inside electrolyzers to make the process work efficiently. That catalyst is nearly always iridium, a metal so rare that global mining produces less than 10 tonnes annually. Compare that to cobalt, which is produced at more than 100,000 tonnes each year, and you can see why this problem has held back green hydrogen’s promise.
The Iridium Barrier
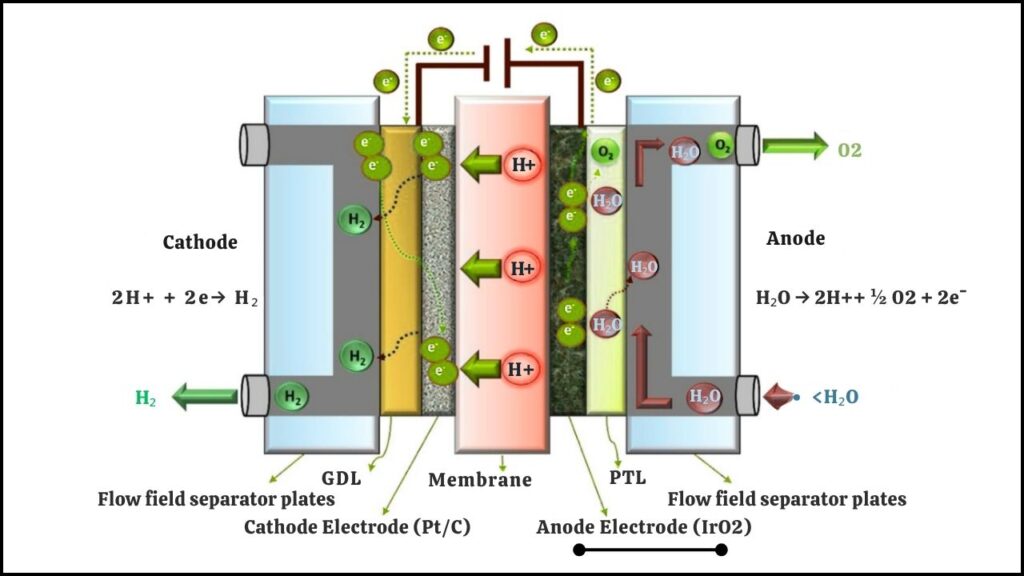
Proton-exchange membrane (PEM) electrolyzers are the most advanced machines for making green hydrogen. They’re efficient, compact, and fast—ideal for pairing with intermittent renewable energy sources. But these devices rely on iridium at the anode to catalyze the crucial water-splitting reaction. Beyond being expensive and scarce, iridium’s limited availability means that, even if you wanted to, you couldn’t build enough electrolyzers to meet the world’s decarbonization goals. This is a major barrier to the clean energy transition.
Cobalt: The Affordable Alternative
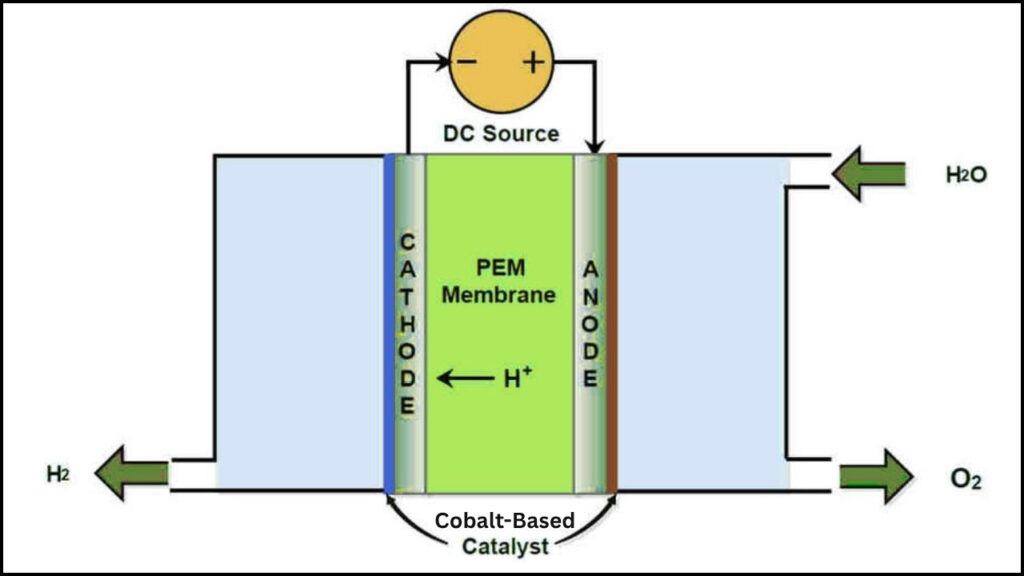
Cobalt is a metal that’s much more common, less expensive, and much less of a risk for supply shortages than iridium. In theory, cobalt-based catalysts could be the answer to the green hydrogen cost problem. For years, scientists have tried to use cobalt in PEM electrolyzers, but the metal simply wore out too quickly—it couldn’t survive the harsh, acidic conditions inside the device. This meant that, although cobalt was cheap, it wasn’t practical for real-world hydrogen production. Until now.
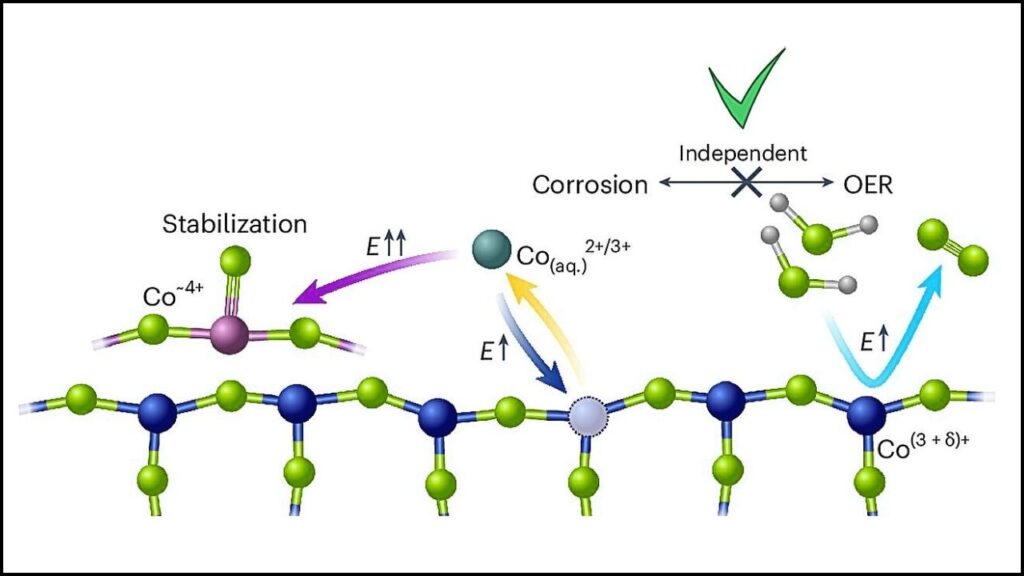
The Monash-led international team found the missing piece: catalyst activity and degradation aren’t directly linked. This is a paradigm shift. It means engineers can now focus on making a cobalt catalyst that’s both highly effective and very stable—two things that were once thought to be in conflict, but are now known to be independent of each other.
How Scientists Cracked the Cobalt Code
This wasn’t a simple experiment. The Monash team, along with collaborators in Germany, the United States, the United Kingdom, and Australia, spent over three years using some of the world’s most advanced spectroscopic, electrochemical, and computational techniques. They watched cobalt catalysts in action at the atomic level, inside working electrolyzers, to see exactly how—and why—they degraded.
What they found was a surprise: the processes that make the catalyst work (its activity) and the processes that cause it to break down (its degradation) happen separately. This means you can engineer for maximum performance and separately engineer for maximum durability—something that was previously seen as impossible.
“Essentially, we’ve uncovered that these processes run in parallel rather than being directly linked,” explained Associate Professor Rosalie Hocking from Swinburne University of Technology. “That gives us a clear pathway to making cobalt-based anodes robust and economically viable for green hydrogen production.” This insight could also help improve other catalyst systems beyond hydrogen, opening the door to even more clean energy innovations.
The work was supported by Monash University, the Australian Research Council, the Australian Renewable Energy Agency, the Office of Naval Research Global (U.S.), the German Academic Exchange Service, and the German Federal Ministry of Education and Research, highlighting the international importance of this breakthrough.
Step-by-Step: How This Breakthrough Unlocks Cheap Green Hydrogen
1. The Challenge
- Iridium is too rare and expensive for global green hydrogen production.
- Cobalt is a promising alternative, but its catalysts have always degraded too quickly in electrolyzers.
2. The Roadblock
- Previous attempts failed because engineers believed catalyst activity and stability were linked—making one better made the other worse.
- Real-world use was impossible because cobalt-based anodes couldn’t last long enough.
3. The Discovery
- Monash scientists discovered that catalyst activity and degradation are independent.
- This is a new concept in catalyst design, and it changes everything.
4. The Path Forward
- Engineers can now optimize each property separately: make cobalt catalysts highly active, and then make them highly durable.
- This could remove the iridium bottleneck and enable mass production of green hydrogen.
5. Broader Impact
- The same approach could work for other clean energy catalysts.
- Global decarbonization becomes more realistic as green hydrogen scales up and costs come down.
What This Means for You
For Students and Young Learners
Think of this like finding out that, with a little tweaking, you can use a cheap, ordinary battery for your robot instead of a rare, expensive one—and it might even work better! This means clean energy could become as cheap and easy as fossil fuels, giving everyone a better chance at a safer climate.
For Professionals and Industry
This is a big deal for the energy sector. Cost is the main obstacle to green hydrogen’s growth. With cheap, abundant cobalt replacing iridium, the economics of large-scale green hydrogen projects look much better. Invest in hydrogen infrastructure, batteries, and related technologies—this is a sector ready to explode.
For Policymakers
Support green hydrogen research, manufacturing, and deployment. If Australia, Germany, the U.S., and other partners can commercialize this technology, it will create jobs, boost energy security, and help meet international climate goals. Now is the time for smart planning and smart investment.
Practical Advice and Next Steps
- If you work in energy or manufacturing: Start exploring partnerships with materials scientists and companies focused on hydrogen tech.
- If you’re an investor: Pay attention to startups and established firms commercializing cobalt-based electrolyzers.
- If you’re an educator: Use this story to inspire students—show them how science and technology can solve global problems.
- If you’re just a concerned citizen: Advocate for clean energy policies and support companies that are investing in green hydrogen.
Cobalt-Based Catalyst Offers an Effective Alternative to Precious Metals
Precisely Placed Platinum Atoms Boost Catalyst Efficiency and Speed
Seaweed-Infused Cement: A Game-Changer for Greener, Stronger Concrete
FAQs About Monash Breakthrough
What is green hydrogen?
Green hydrogen is hydrogen gas made by splitting water using electricity from renewable sources like wind or solar. Unlike traditional hydrogen, it doesn’t cause climate change.
Why is iridium a problem?
Iridium is a rare and expensive metal used as a catalyst in the best electrolyzers. There simply isn’t enough iridium to make green hydrogen at the scale needed to replace fossil fuels.
How does cobalt help?
Cobalt is much more abundant and affordable than iridium. If cobalt-based catalysts can be made stable and effective, they can replace iridium, making green hydrogen cheaper and scalable.
Why was cobalt unstable in electrolyzers before?
Cobalt catalysts broke down quickly in the harsh, acidic conditions inside the electrolyzer, losing their effectiveness in just hours or days.
What did the Monash team discover?
They found that the process that makes the cobalt catalyst work (its activity) and the process that makes it degrade (its stability) are separate, not linked. This means you can work to improve both independently.
When will we see cobalt-based electrolyzers?
The research is very recent, but the findings are clear. It may take a few years of engineering and scale-up before these catalysts are used in commercial electrolyzers, but progress is moving quickly.
Who funded this research?
The work was supported by Monash University, the Australian Research Council, the Australian Renewable Energy Agency, the U.S. Office of Naval Research Global, and German government agencies, among others.