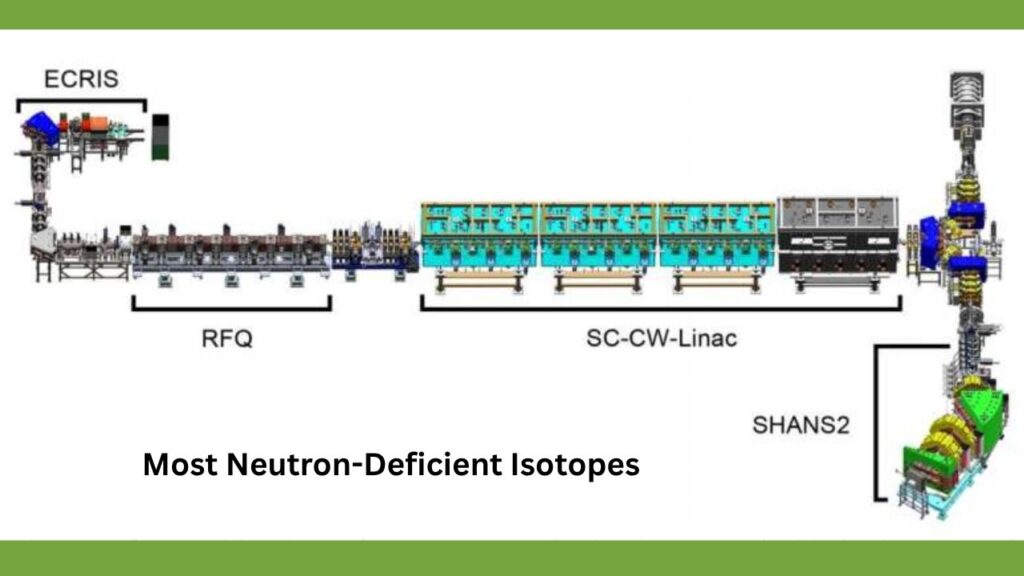
Most Neutron-Deficient Isotopes Ever Observed in the Lab: Physicists have recently created one of the most neutron-deficient isotopes ever observed in laboratory settings, marking a major breakthrough in nuclear science. These exotic isotopes push the boundaries of our understanding of atomic nuclei, nuclear stability, and the fundamental forces that govern matter. The creation of neutron-deficient isotopes provides vital clues about how nuclei behave when stripped of many neutrons, which can reveal the limits of nuclear stability and deepen our knowledge of elemental formation in the universe.
This article will comprehensively explore what neutron-deficient isotopes are, how they are synthesized, why their discovery is significant, and what implications this has for science and technology. We will also provide practical insights for students, researchers, and enthusiasts interested in the fascinating field of nuclear physics.
Most Neutron-Deficient Isotopes Ever Observed in the Lab
| Feature | Actinium-203 (Ac-203) | Uranium-214 (U-214) |
|---|---|---|
| Neutron Count | Extremely Neutron-Deficient | Lightest Uranium Isotope |
| Proton Number | 89 (Actinium) | 92 (Uranium) |
| Half-Life | 56 microseconds | Very short (exact value measured) |
| Alpha-Particle Energy | 8217 keV | Detected via decay spectroscopy |
| Production Method | Fusion-evaporation with Ca-40 beam | Bombardment with Ar and Ca ions |
| Production Cross-Section | 0.13 picobarns | Very low, indicating rarity |
| Research Institution | Institute of Modern Physics, CAS | Chinese Academy of Sciences |
| Implications | Nuclear structure, astrophysics | Limits of nuclear stability |
| Official Reference | Institute of Modern Physics |
The synthesis of neutron-deficient isotopes such as actinium-203 and uranium-214 exemplifies the relentless progress in nuclear physics. These isotopes challenge existing theories about nuclear stability and expand the known boundaries of the nuclear landscape. The insights gained pave the way for enhanced models of atomic nuclei, deepen our understanding of cosmic element formation, and potentially foster future technological innovations.
As experimental capabilities continue to evolve, physicists will keep pushing these boundaries, revealing ever more about the fundamental nature of matter.
Understanding Neutron-Deficient Isotopes: The Basics
Before diving into the latest discoveries, it’s essential to understand some fundamental concepts.
What Are Isotopes?
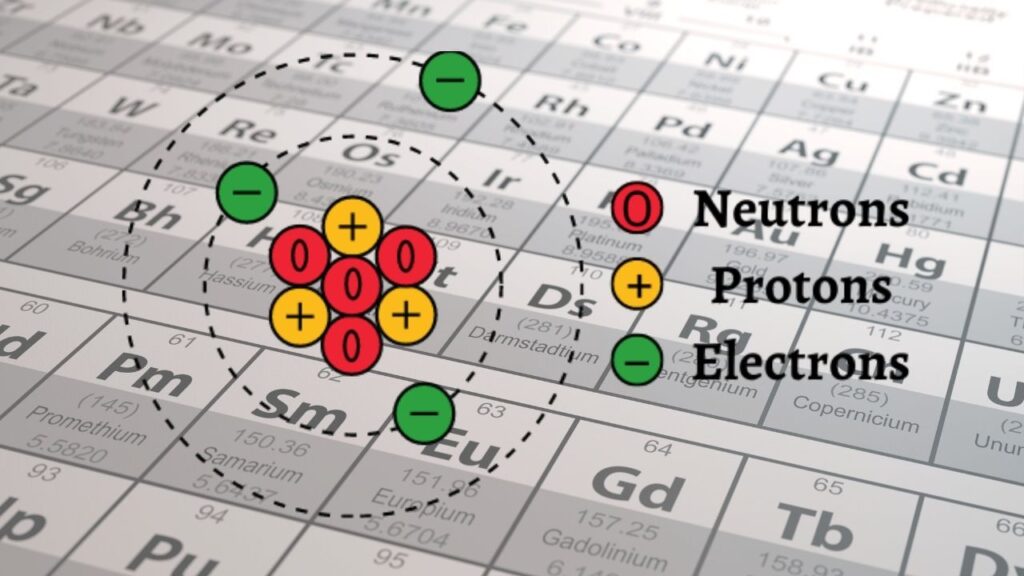
Atoms consist of protons, neutrons, and electrons. While the number of protons (also called the atomic number) defines the chemical element, atoms of the same element can differ in their number of neutrons — these variations are called isotopes.
- Stable isotopes have neutron numbers that allow the nucleus to remain intact for long periods.
- Unstable or radioactive isotopes have too many or too few neutrons, making their nuclei prone to decay into other elements.
Neutron-Deficiency Explained
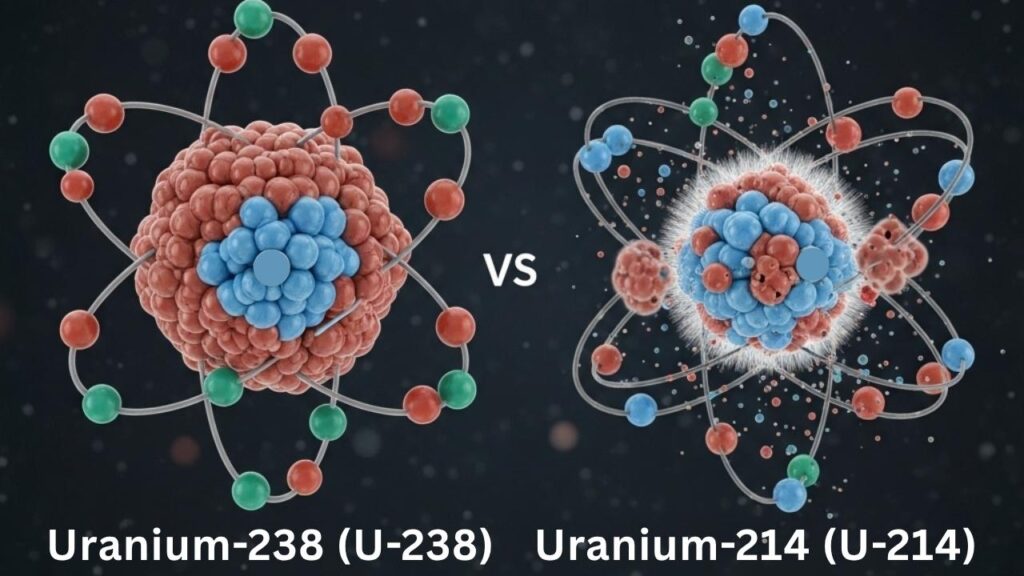
- A neutron-deficient isotope has significantly fewer neutrons than the stable isotopes of the same element.
- Such isotopes tend to be highly unstable because neutrons help offset the repulsive forces between protons.
- Investigating neutron-deficient isotopes helps scientists understand nuclear forces, decay mechanisms, and the “drip lines”—the boundaries beyond which nuclei cannot hold additional protons or neutrons.
For example, uranium-238 (U-238), the most common isotope of uranium, contains 92 protons and 146 neutrons. The newly created uranium-214 (U-214) isotope contains the same 92 protons but only 122 neutrons, representing a neutron-deficiency that challenges conventional understanding.
How Physicists Create Neutron-Deficient Isotopes
Creating such exotic isotopes requires highly sophisticated experimental setups, often found only in advanced nuclear physics laboratories worldwide. Here’s a detailed look at the methodology:
Step 1: Accelerating Ions
Physicists use particle accelerators, like cyclotrons or linear accelerators, to speed up charged particles (ions) to extremely high energies.
- Common projectiles include isotopes like calcium-40 (^40Ca) or argon-36 (^36Ar).
- The accelerated ions gain kinetic energy sufficient to overcome electrostatic repulsion and fuse with target nuclei.
Step 2: Target Bombardment and Fusion
The high-energy ion beam bombards a target material composed of heavy nuclei, such as tungsten (W) or thorium (Th).
- The collisions produce a compound nucleus, a temporary merged system.
- This compound nucleus is highly excited and quickly “cools” by evaporating excess particles (usually neutrons, protons, or alpha particles).
- If fewer neutrons are emitted, the resulting nucleus ends up neutron-deficient.
Step 3: Isolation and Identification
Because these neutron-deficient isotopes are extremely unstable—existing for microseconds to milliseconds—scientists must detect their decay signatures quickly and accurately.
- Detection techniques include alpha-decay spectroscopy and mass spectrometry.
- Specialized detectors measure emitted particles’ energy, timing, and trajectories.
- The unique decay patterns confirm the identity and properties of the new isotope.
Step 4: Data Analysis and Verification
- Extensive analysis verifies that the isotope observed matches predictions.
- Cross-section measurements (which quantify production probability) are made, often showing the rarity of these isotopes.
Recent Breakthroughs: Actinium-203 and Uranium-214
Two neutron-deficient isotopes that have recently been synthesized highlight the rapid progress in this field.
Actinium-203 (Ac-203)
- Produced by: The Institute of Modern Physics (IMP), Chinese Academy of Sciences.
- Method: Bombarding a target with ^40Ca beams via fusion-evaporation reactions.
- Properties:
- Proton number: 89 (actinium)
- Alpha-particle energy: 8217 keV
- Half-life: ~56 microseconds
- Production cross-section: 0.13 picobarns (extremely low, indicating rarity)
- Significance: This isotope lies near the proton drip line, testing nuclear models about stability and decay modes.
Uranium-214 (U-214)
- Produced by: A team at the Chinese Academy of Sciences.
- Method: Bombarding tungsten targets with argon and calcium ions.
- Properties:
- Proton number: 92 (uranium)
- Neutron number: 122 (neutron-deficient compared to stable isotopes)
- Very short half-life, detected through decay spectroscopy.
- Significance: The lightest uranium isotope ever observed, expanding the known nuclear landscape for heavy elements.
Why These Discoveries Matter: The Broader Impact
The synthesis of neutron-deficient isotopes is not just a technical tour de force — it carries substantial scientific and practical importance.
Testing and Improving Nuclear Models
- Theoretical models like the nuclear shell model and liquid drop model predict nuclear stability and magic numbers.
- These newly synthesized isotopes provide data points at the edges of stability, helping refine predictions about nuclear forces and decay mechanisms.
Insights into Astrophysical Processes
- Neutron-deficient isotopes help us understand nucleosynthesis pathways in extreme environments, such as supernovae and neutron star mergers.
- The rp-process (rapid proton capture) involves proton-rich nuclei and is critical for explaining the creation of certain elements in the cosmos.
Applications in Nuclear Medicine and Technology
- Radioisotopes are used in diagnostics and therapy (e.g., cancer treatments).
- Although neutron-deficient isotopes like Ac-203 and U-214 are currently too unstable for direct applications, studying their properties aids the development of medical isotopes.
Fundamental Science and Element Discovery
- Exploring the limits of nuclear existence aids in the discovery of new elements and isotopes.
- Understanding neutron deficiency helps predict where the “proton drip line” lies — beyond which nuclei cannot hold protons, defining the edge of nuclear stability.
Practical Advice for Aspiring Nuclear Physicists
For those interested in joining this cutting-edge field, here is a practical guide:
Step 1: Build a Strong Foundation
- Study physics fundamentals, focusing on nuclear physics.
- Recommended reading: Introductory Nuclear Physics by Kenneth Krane.
Step 2: Learn Experimental Techniques
- Gain hands-on experience with particle accelerators, detectors, and data analysis.
- Explore internships or research projects at national labs or universities.
Step 3: Understand Theoretical Models
- Delve into nuclear models: shell model, collective model, and density functional theory.
- Engage with computational tools for simulating nuclear reactions.
Step 4: Stay Updated with Research
- Read journals such as Physical Review C and Nuclear Physics A.
- Follow conferences like the International Nuclear Physics Conference (INPC).
Step 5: Collaborate and Innovate
- Network with experts.
- Propose experiments or theoretical investigations into neutron-deficient isotopes.
Rice University Researchers Discover New 2D Material With Exceptional Adhesion Properties
ISRO Declares 2025 as Gaganyaan Year with December Launch Featuring Humanoid Robot Vyommitra
Berkeley Lab Unveils Doudna Supercomputer to Propel AI and Genomics Research
FAQs About Most Neutron-Deficient Isotopes Ever Observed in the Lab
Q1: How do neutron-deficient isotopes differ from neutron-rich ones?
A: Neutron-deficient isotopes have fewer neutrons than stable forms, making them proton-rich and often highly unstable. Neutron-rich isotopes have extra neutrons and exhibit different decay modes.
Q2: Why are these isotopes so short-lived?
A: Instability arises from an imbalance in nuclear forces; without enough neutrons to balance proton repulsion, the nucleus quickly undergoes radioactive decay to achieve a more stable state.
Q3: What challenges exist in detecting neutron-deficient isotopes?
A: Their short half-lives (microseconds to milliseconds) require fast, sensitive detection equipment and precise experimental setups.
Q4: Could neutron-deficient isotopes be useful in medicine?
A: While direct use is limited due to instability, understanding their properties aids development of other medically relevant isotopes.
Q5: Where can I find more detailed nuclear data?
A: Trusted databases include the National Nuclear Data Center (NNDC) and the IAEA Nuclear Data Services.