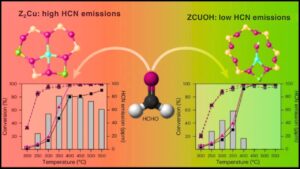
Biodiesel is a leading sustainable alternative to fossil fuels, but one of its production challenges has been the catalysts involved in the chemical conversion of oils into fuel. Traditionally, these catalysts contain metals which can be costly, toxic, and environmentally harmful. However, new advances in metal-free carbon-based catalysts now promise to transform biodiesel production by making it greener, cheaper, and more efficient.
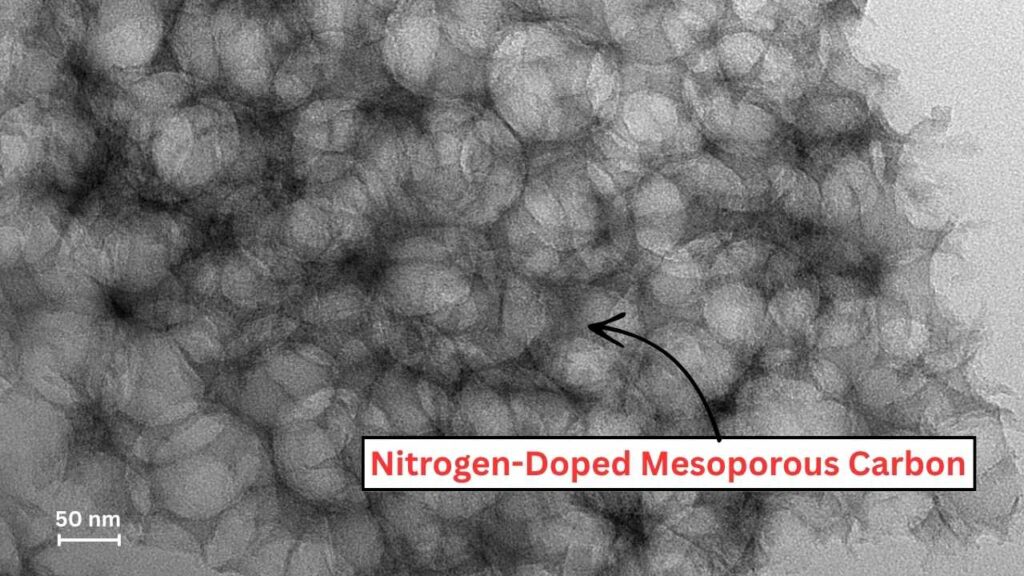
This article explores how metal-free carbon catalysts derived from biomass offer a revolutionary approach to producing eco-friendly biodiesel. We will break down the science behind these catalysts, their advantages, practical steps for biodiesel synthesis using these materials, and why they signify a major leap forward in renewable energy.
Table of Contents
What Is Biodiesel and Why Does Catalyst Matter?
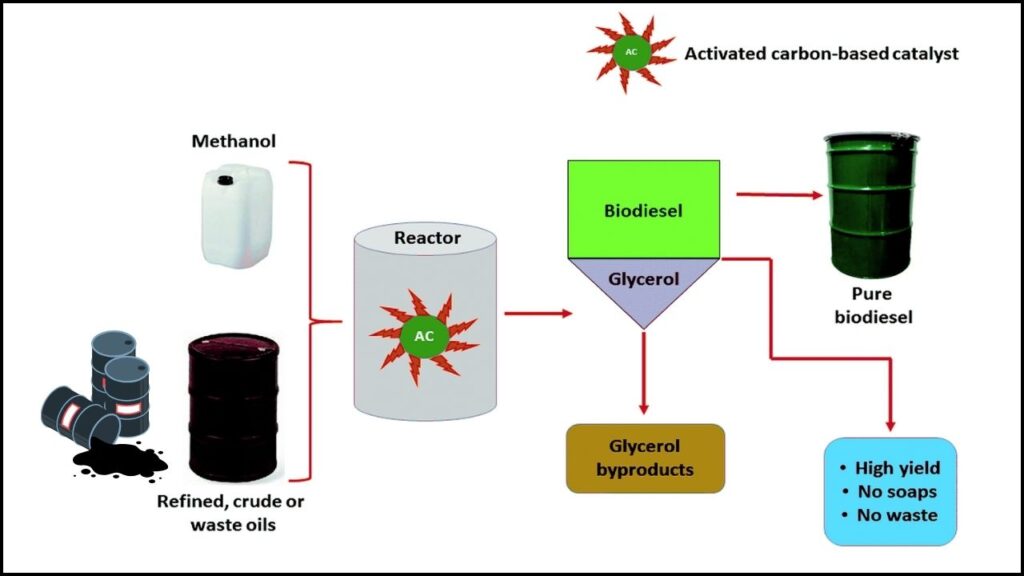
Biodiesel is a renewable fuel produced by processing natural oils and fats through a chemical reaction called transesterification. During this process, fats react with an alcohol (usually methanol) in the presence of a catalyst to produce biodiesel and glycerol as a byproduct.
Catalysts are substances that speed up this chemical reaction without being consumed. Traditionally, metal-based catalysts such as sodium hydroxide and potassium hydroxide have been used in biodiesel manufacturing because they are effective. However, these come with drawbacks:
- They can be toxic and corrosive, requiring careful handling.
- Their use leads to hazardous waste, increasing environmental concerns.
- Catalysts need to be neutralized and washed out post-reaction, raising operational costs.
- Some metals used are expensive or scarce, limiting sustainability.
Enter Metal-Free Carbon Catalysts
Researchers have developed catalysts based purely on activated carbon materials derived from natural biomass waste, such as:
- Date palm fronds
- Bean husks
- Agricultural residues
These carbon-based catalysts undergo specific treatments to enhance their surface area and introduce functional groups like nitrogen doping or sulfonic acid groups, which provide catalytic activity without the need for metals.
That’s key: these catalysts do not rely on metals, so they avoid toxicity and environmental harm associated with metal waste. They are solid, reusable, and cost-effective, making biodiesel production cleaner and more sustainable.
New Carbon Catalyst Could Revolutionize Biodiesel
| Feature | Details |
|---|---|
| Catalyst Type | Metal-free, nitrogen-doped mesoporous carbon and activated carbon derived from biomass |
| Feedstocks | Waste cooking oil, vegetable oils, residual biomass (e.g., bean husks, palm fronds) |
| Biodiesel Yield | Up to 95-97% ester content under optimal conditions |
| Reaction Conditions | 80–120 °C, methanol:oil molar ratio 10:1 to 20:1, catalyst loading 5–8 wt%, 2–3 hours |
| Environmental Impact | Non-toxic, recyclable, valorizes agricultural waste, reduces harmful effluents |
| Professional Application | Scalable and sustainable biodiesel manufacturing with reduced cost and waste handling |
The development of metal-free carbon catalysts derived from biomass waste marks a significant milestone in biodiesel production. These catalysts are not only highly efficient, delivering biodiesel yields up to 95-97%, but they also eliminate toxic metals, reduce costs, and promote circular bioeconomy principles by using agricultural residues. As a result, they offer a promising, sustainable path to produce clean energy fuels at scale, aligning perfectly with today’s environmental and economic demands.
For anyone interested in sustainable biofuel technologies, exploring carbon catalysts represents a smart, responsible choice toward a cleaner energy future.
The Science Behind Carbon Catalysts
Unlike homogeneous liquid catalysts which dissolve into the reaction mixture, carbon catalysts are heterogeneous solids — meaning they are solid particles that do not dissolve but provide surfaces where reactions happen.
How Are These Catalysts Made?
- Biomass as Carbon Source: Agricultural waste like bean husks or palm fronds is collected.
- Activation Process: Through heating at high temperatures (~400-800 °C) without oxygen (calcination), these materials are converted into activated carbon.
- Functionalization: Chemical treatments add active groups (e.g., sulfonic acid or nitrogen-doping) to catalyze the transesterification reaction efficiently.
- Characterization: Techniques such as X-ray diffraction and scanning electron microscopy verify the catalysts’ surface features and composition.
How Do Carbon Catalysts Work?
The porous structure of activated carbon provides a vast surface area for reacting molecules to interact. The functional groups attached to the carbon surface act as active sites, attracting and converting the fats and methanol into biodiesel.
Because they don’t contain metals, these catalysts avoid metal leaching, which reduces environmental contamination and allows easy recovery and reuse, lowering production costs.
Practical Guide to Biodiesel Production Using Metal-Free Carbon Catalysts
Whether you are an industrial producer or an enthusiast looking to explore sustainable biofuel production, here is a straightforward guide:
Step 1: Choose Your Feedstock
Ideal feedstocks include:
- Waste cooking oils — readily available and low-cost
- Vegetable oils such as soybean or canola oil
- Animal fats or specialized non-food biomass oils
Step 2: Prepare or Acquire Carbon Catalyst
Carbon catalysts prepared from biomass waste are increasingly commercially available. If making your own, biomass is activated by controlled heating and functionalized to optimize activity.
Step 3: Define Reaction Parameters
- Methanol to oil molar ratio: 10:1 to 20:1
- Catalyst loading: Around 5-8 percent by weight of oil
- Temperature: Maintain between 80 °C to 120 °C
- Reaction time: 2 to 3 hours with continuous stirring
Step 4: Conduct the Reaction
Mix the oil, methanol, and catalyst under the set conditions. The catalyst will speed up the breakdown of oils to biodiesel and glycerol.
Step 5: Separate Products
Once complete, allow the mixture to settle so biodiesel separates from glycerol. Filter to recover the catalyst for reuse.
Step 6: Purify and Test
Wash and dry the biodiesel to remove impurities and test for quality standards (e.g., viscosity, ester content).
Why This Breakthrough Matters
Environmental Benefits
- Less Toxic Waste: Avoiding metal catalysts reduces harmful effluents.
- Waste Valorization: Carbon catalysts come from agricultural residues, turning waste into valuable products.
- Lower Carbon Footprint: Efficient catalysts reduce energy consumption and emissions.
Economic Advantages
- Cost Cutting: Using low-cost biomass feedstocks and recyclable catalysts slashes expenses.
- Simplified Processing: Solid catalysts simplify separation and purification stages.
- Scalability: Catalysts are easy to produce on a large scale from abundant natural materials.
Future Potential
With ongoing research improving catalyst stability and activity, wider commercialization is imminent. The approach aligns with global sustainability goals and supports energy independence from fossil fuels.
Engineered Ruthenium Catalysts: A Sustainable Revolution for Hydrogen and Ammonia
New Triple-Layer Catalyst Boosts Green Hydrogen Output by 800%—A Game-Changer for Clean Energy
Breakthrough Catalyst Efficiently Converts CO₂ Into Methanol With High Precision
FAQs About New Carbon Catalyst Could Revolutionize Biodiesel
What are the main advantages of metal-free carbon catalysts over traditional ones?
Metal-free carbon catalysts are non-toxic, reusable, derived from waste biomass, cost-effective, and reduce harmful emissions associated with metal catalysts.
Can waste cooking oil be directly used in this process?
Yes, but pre-treatment such as filtering and drying improves catalyst performance by removing moisture and impurities.
How many times can the carbon catalyst be reused?
Most studies show successful reuse for multiple cycles (at least 3-4) with only minor decrease in efficiency.
What makes biomass-derived carbon catalysts effective?
Their high surface area, porous structure, and active functional groups (like sulfonic acids or nitrogen sites) provide abundant reactive sites to facilitate biodiesel formation.
Is this technology available commercially?
While still advancing, several companies and research groups are scaling up production of biomass-based carbon catalysts for industrial biodiesel plants.