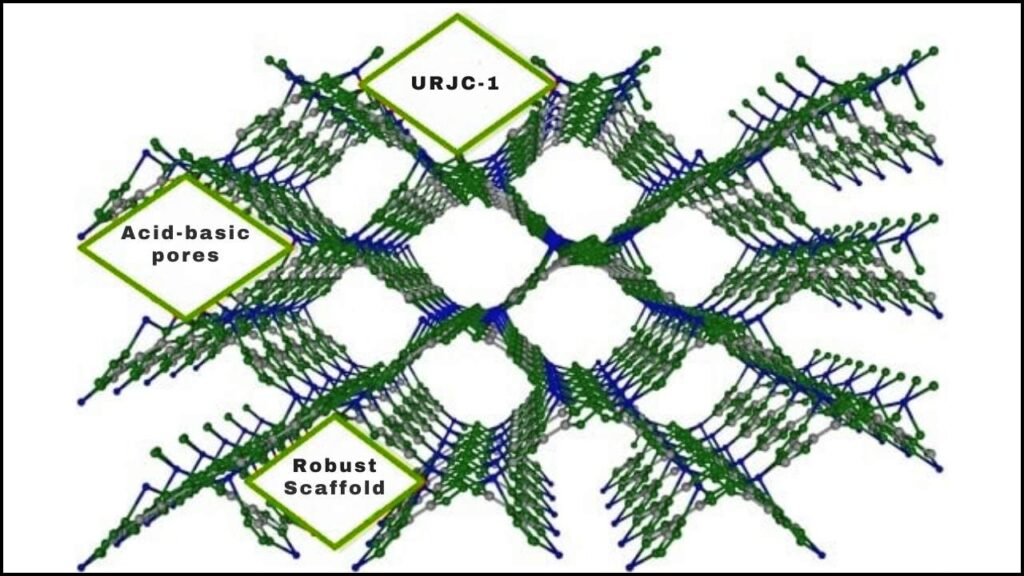
Metal–organic frameworks, commonly known as MOFs, have become a groundbreaking tool in the world of chemistry. Recently, a remarkable new MOF catalyst named URJC-1 has been developed to make C–O cross coupling reactions more efficient, faster, and environmentally friendly. This advancement promises significant benefits for the pharmaceutical industry, material science, and green chemistry, making complex chemical manufacturing simpler and more sustainable.
This article will explain the concept and importance of MOF catalysts, the role of C–O cross coupling reactions in everyday products, and how this new URJC-1 catalyst is changing the game — all in a clear, approachable style. Whether you’re a student, chemist, or just curious about innovative science, this guide covers all you need to know with practical examples and easy-to-follow explanations.
Table of Contents
New MOF Catalyst Developed for Efficient C–O Cross Coupling Reactions
| Aspect | Details |
|---|---|
| Catalyst Name | URJC-1, a copper-based Metal-Organic Framework (MOF) |
| Reaction Type | C–O Cross Coupling (O-arylation) |
| Catalyst Loading | Low, 3 mol% copper |
| Temperature Required | 120°C |
| Reaction Time | 1 hour |
| Stability | Maintains crystalline structure after multiple uses |
| Yield | Complete conversion with 100% selectivity |
| Industrial Applications | Pharmaceuticals, polymers, fine chemicals |
| Mechanism | Combination of Lewis acid copper centers and Lewis base nitrogen atoms on the organic ligand |
| Official Reference Website | PubMed Central Article on URJC-1 MOF Catalyst |
The development of the URJC-1 MOF catalyst marks a significant advance in making C–O cross coupling reactions more efficient, economical, and eco-friendly. Its dual-action mechanism and robust structure enable fast, selective reactions under mild conditions and allow easy recycling — a powerful combination for sustainable chemistry.
For manufacturers and researchers looking to improve synthesis routes in pharmaceuticals, polymer chemistry, or fine chemicals, URJC-1 offers a reliable catalyst that balances cost, performance, and environmental impact.
What Are Metal-Organic Frameworks (MOFs)?
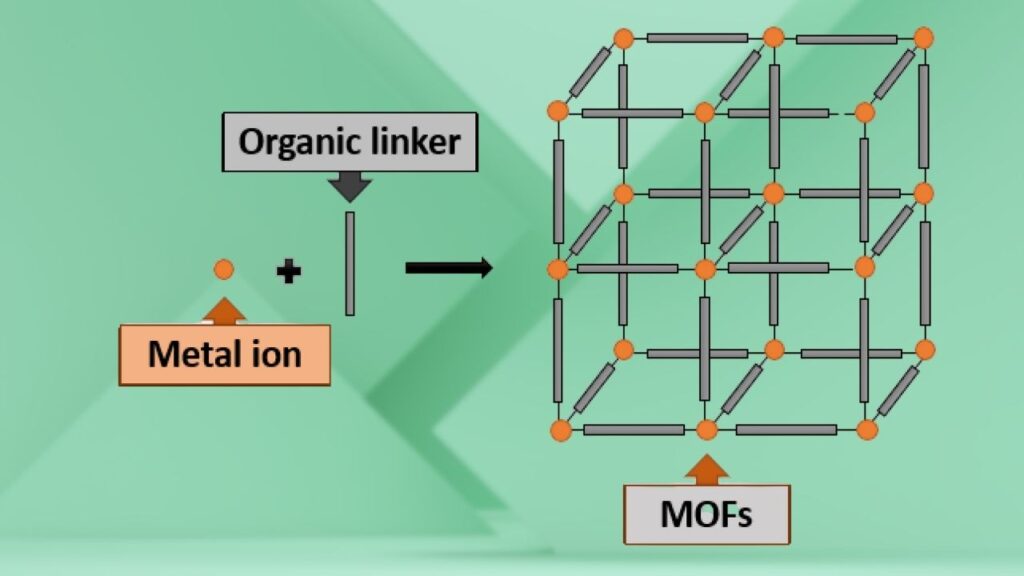
Imagine building a tiny, incredibly strong scaffold using metallic atoms as joints and organic molecules as connecting beams — that’s essentially what a metal-organic framework (MOF) is. These frameworks are porous, meaning they have lots of tiny holes inside, allowing them to trap and transform molecules during chemical reactions.
The amazing thing about MOFs is their customizable design. Scientists can change the metal atoms or the organic linkers depending on the specific task, making MOFs like tailor-made catalysts. MOFs can speed up chemical reactions, hold gases, filter pollutants, and even capture carbon dioxide.
Why Are C–O Cross Coupling Reactions Important?
In organic chemistry, cross coupling reactions help us join two building blocks to create new and useful molecules. The carbon-oxygen (C–O) bond formation is especially important because it’s found in many valuable products like:
- Medicines (active pharmaceutical ingredients)
- Agrochemicals (for crop protection)
- Polymers and plastics
- Fine chemicals used in everyday products
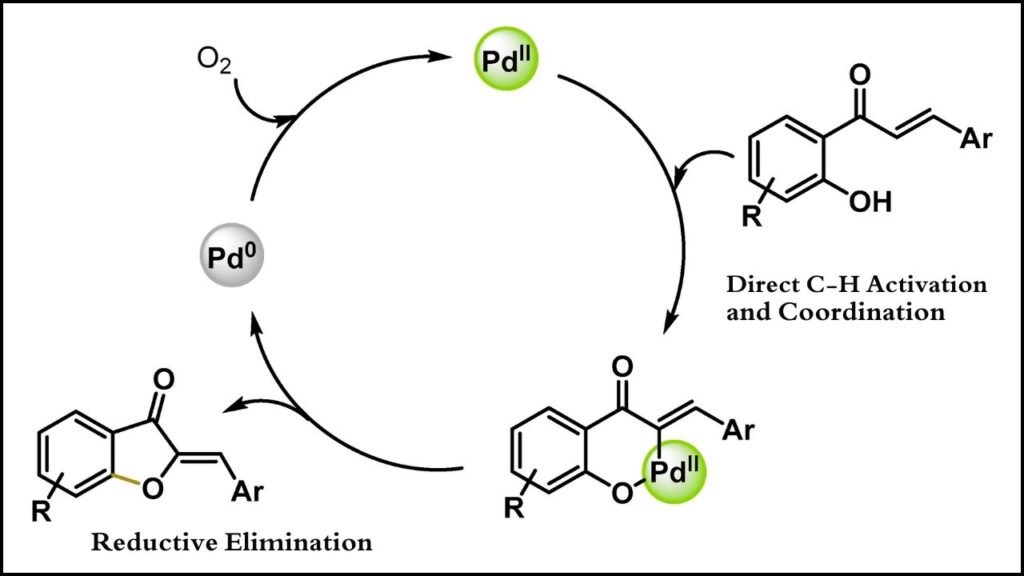
Traditionally, C–O cross coupling reactions required precious metals like palladium, which are expensive and sometimes less environmentally friendly.
The Breakthrough: URJC-1 MOF Catalyst
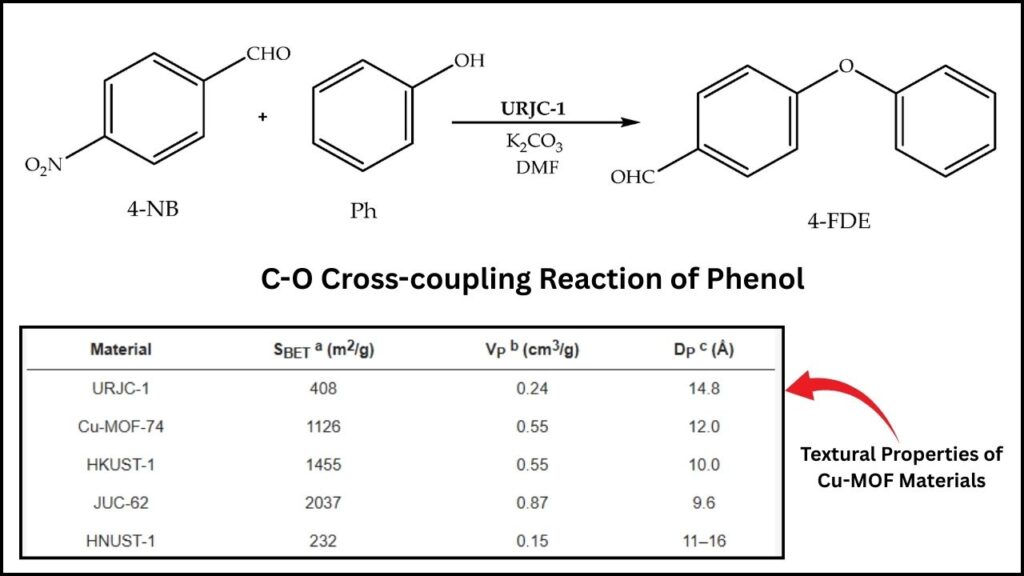
The URJC-1 catalyst is a revolutionary copper-based MOF structured with special organic molecules containing nitrogen atoms from imidazole and tetrazole rings. This unique combination creates dual active sites within the catalyst:
- Copper centers act as Lewis acids (electron attractors),
- Nitrogen atoms on the organic ligands act as Lewis bases (electron donors).
This dual action helps the catalyst effectively join phenols (compounds containing an -OH group) with aryl halides (carbon-halogen molecules) to form diaryl ethers, a valuable class of compounds.
What makes URJC-1 stand out?
- It needs only 3% mol copper catalyst which is low compared to traditional methods.
- Achieves complete conversion in just 1 hour at a moderate temperature of 120°C.
- Exhibits excellent selectivity, meaning it creates the desired product with virtually no by-products.
- Shows remarkable chemical and thermal stability, remaining intact even after several reaction cycles.
- Is recyclable, meaning it can be reused many times without losing activity, reducing both cost and environmental impact.
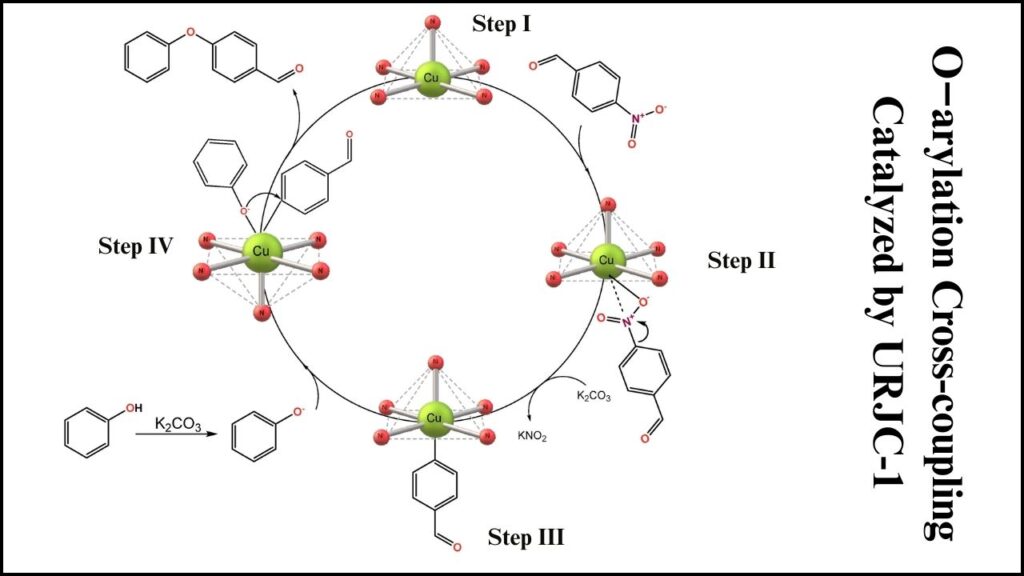
How Does the URJC-1 Catalyst Work?
The catalytic process can be summarized in a few key steps:
- Activation of the reactants: The copper centers activate the aryl halide by coordinating with the halogen molecule.
- Nucleophilic attack: The phenol’s oxygen attacks the activated carbon, facilitated by the Lewis base sites on nitrogen atoms enhancing its reactivity.
- Coupling and product formation: This results in the formation of a new C–O bond, yielding a diaryl ether.
- Catalyst regeneration: The URJC-1 structure remains unchanged, ready to catalyze the next reaction cycle.
This synergy between metal and organic parts within the MOF framework makes the reaction faster and more efficient than many existing catalysts.
Step-by-Step Guide to Using URJC-1 for C–O Cross Coupling
- Gather Materials: The reagents needed include an aryl halide, the phenol partner, a base like potassium carbonate (K₂CO₃), and the URJC-1 catalyst.
- Prepare Reaction Mixture: Mix the aryl halide, phenol, base, and 3 mol% of URJC-1 catalyst in a suitable solvent such as dimethylformamide (DMF).
- Heat the Reaction: Maintain the mixture at 120°C under constant stirring.
- Monitor the Reaction: The reaction typically completes within 1 hour, forming the desired diaryl ether.
- Separate Catalyst: The solid MOF catalyst can be filtered out easily after the reaction.
- Recycle Catalyst: After washing and drying, the URJC-1 catalyst can be reused for multiple cycles without loss of function.
- Isolate Product: Purify the reaction product using standard techniques like extraction or chromatography.
Practical Tips for Chemists and Researchers
- Store URJC-1 catalyst in a dry environment to maintain its high activity.
- Ensure proper temperature control for consistent results.
- Test various substrates with activating substituents (like methoxy or chloro groups) for better conversion.
- Be aware that bulkier molecules may react slower due to steric hindrance within the MOF pores.
- Follow safety protocols when handling reagents and solvents.
New Low-Cost Catalyst Discovered for Clean Hydrogen Energy: A Game-Changer for the Future
Breakthrough Catalysts for Diphenylamine Alkylation Using Citric Acid-Dispersed Ce on MCM-22
FAQs About New MOF Catalyst Developed for Efficient C–O Cross Coupling Reactions
Q: What makes MOF catalysts better than traditional ones?
MOFs offer high surface areas, tunable active sites, and easy recyclability. Unlike many traditional catalysts, they combine multiple functions within one structure and withstand harsh reaction conditions without degradation.
Q: Are copper-based MOFs as effective as palladium catalysts?
While palladium is often more active, copper-based MOFs like URJC-1 are cost-effective, abundant, and environmentally friendlier with competitive activity and stability for many reactions.
Q: Can URJC-1 be reused?
Yes, URJC-1 maintains its structure and activity after multiple reaction cycles, reducing waste and cost.
Q: What industries benefit from this catalyst?
Pharmaceutical, agrochemical, polymer, and fine chemical industries can all leverage this catalyst for creating complex molecules more sustainably.
Q: Does URJC-1 work with a variety of substrates?
Yes, URJC-1 is effective with different substituted phenols and aryl halides, especially when activating groups are present.