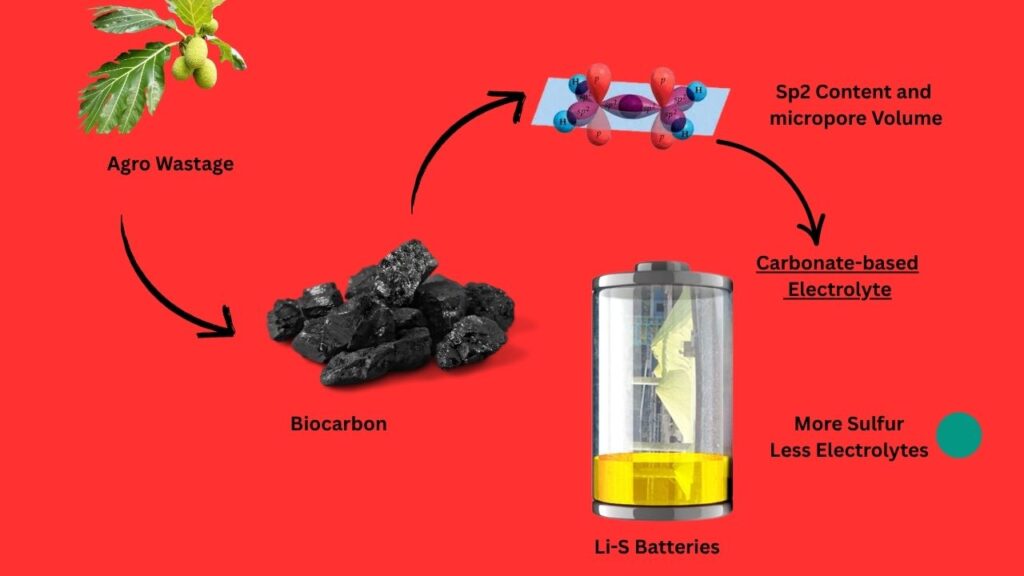
Biocarbon from Agro‑Waste Serves as Sulfur Host in Next‑Gen Li‑S Batteries: If you’re keeping your finger on the pulse of battery tech, you’ve probably heard the buzz about lithium-sulfur batteries. But what’s really turning heads lately is the use of biocarbon from agro-waste as a sulfur host in these next-gen powerhouses. This breakthrough isn’t just science for the sake of science—it’s a game-changer for everything from your smartphone to electric trucks rolling down Route 66.
Let’s break it down, cowboy style—easy enough for a 10-year-old to get, but with the nitty-gritty details pros crave.
Biocarbon from Agro‑Waste Serves as Sulfur Host in Next‑Gen Li‑S Batteries
| Feature/Stat | Details & Data | Career/Professional Info |
|---|---|---|
| Battery Type | Lithium-Sulfur (Li-S) | Engineers, Chemists, Battery Makers |
| Sulfur Host | Biocarbon from agro-waste (e.g., soybean skins, vegetable sponge) | Materials Scientists, Sustainability |
| Specific Capacity | Up to 1377 mAh/g with 66% sulfur loading | Battery Designers, R&D |
| Energy Density | Over 300 Wh/kg (surpasses most Li-ion batteries) | EV Manufacturers, Grid Storage |
| Environmental Impact | Uses agricultural waste, cuts cost, reduces pollution | Sustainability Officers, Policymakers |
| Main Challenge | Managing polysulfide “shuttle effect” for longer battery life | Battery Engineers |
| Commercial Readiness | Not yet mass-market, but rapidly advancing | Investors, Tech Startups |
Biocarbon from agro-waste is flipping the script on battery tech. By turning farm leftovers into high-performance sulfur hosts, we’re getting batteries that are lighter, cheaper, greener, and more powerful than ever. Whether you’re a kid dreaming of electric trucks or a pro designing the next big thing, this technology is set to reshape our world—one charge at a time.
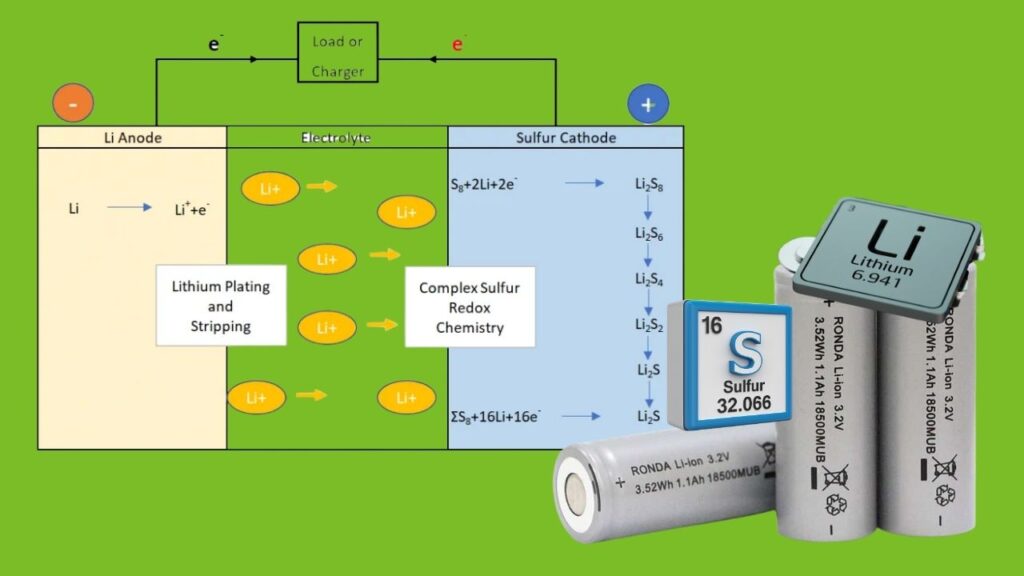
What’s a Lithium–Sulfur Battery, Anyway?
Alright, let’s start at the beginning. Lithium–sulfur (Li–S) batteries are rechargeable batteries that use lithium metal as the negative electrode (anode) and sulfur as the positive electrode (cathode). When you use the battery, lithium ions move from the anode to the cathode, reacting with sulfur and creating energy you can use to power your gadgets or even your ride.
Why all the hype?
- High energy density: Li–S batteries can store way more energy than traditional lithium-ion batteries. We’re talking over 300 Wh/kg, which means lighter, longer-lasting batteries for everything from drones to pickup trucks.
- Low cost and eco-friendly: Sulfur is cheap and everywhere, unlike cobalt or nickel used in regular batteries.
- Green credentials: By using biocarbon from agricultural waste as the sulfur host, we’re turning farm leftovers into high-tech gold.
How Does Biocarbon from Agro-Waste Help?
Here’s where things get cool. Sulfur alone isn’t a great team player—it’s not very good at conducting electricity, and it likes to dissolve away during charging, which can kill your battery fast. Enter biocarbon from agro-waste.
What’s Biocarbon?

Biocarbon is just carbon made by heating up stuff like soybean skins or vegetable sponges left over from farming. This process creates a material with a super-high surface area and lots of tiny holes (pores). Think of it like a sponge for sulfur.
Why is Biocarbon a Game-Changer?
- Holds More Sulfur: The pores in biocarbon trap more sulfur, letting you pack more energy into the battery.
- Stops the “Shuttle Effect”: Normally, when the battery runs, sulfur forms “polysulfides” that can drift around and mess things up (the “shuttle effect”). Biocarbon’s structure keeps these in check, so your battery lasts longer.
- Boosts Conductivity: Carbon conducts electricity, so it helps the battery run smoother and faster.
- Eco-Friendly: We’re talking about upcycling farm waste—good for the planet, good for your wallet.
How Do Lithium–Sulfur Batteries Work? (For Kids and Pros Alike)
Let’s break it down, step by step:
1. Charging Up
- Lithium ions leave the lithium metal anode and travel through the electrolyte to the sulfur cathode.
- They react with sulfur to form lithium polysulfides—think of these as the “middlemen” in the battery.
2. Discharging (Giving You Power)
- The lithium polysulfides get turned into lithium sulfide, releasing energy as they go.
- The battery’s voltage is about 2V—not as high as some, but because it packs in so much energy, it’s still a winner.
3. The “Shuttle Effect” Problem
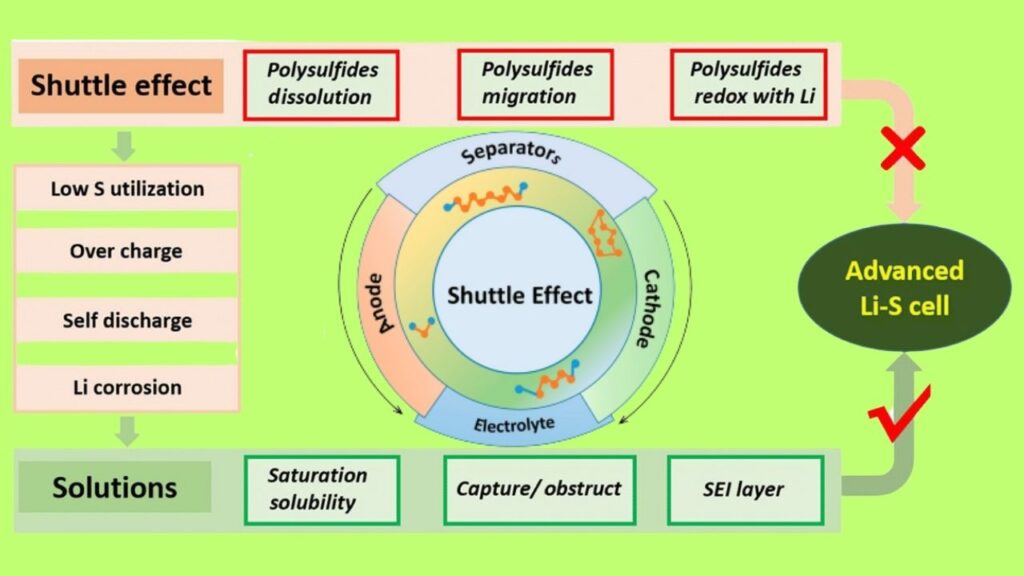
- Some of those polysulfides like to wander off, which can cause the battery to lose capacity over time.
- Biocarbon helps trap those wanderers, keeping the battery running strong.
Real-World Data and Examples
- Soybean Skin Carbon: Researchers made biocarbon from soybean skins with a surface area of 1232 m²/g. They loaded it with 63.7% sulfur, and the battery hit a whopping 1231 mAh/g capacity at 0.5C.
- Vegetable Sponge Carbon: After treating and carbonizing the sponge, it held 63.5% sulfur and delivered 714 mAh/g at 0.2C.
- Cyclosorus Interruptus Carbon: One-step carbonization gave a surface area of 1549.7 m²/g, and with 66.4% sulfur, the battery reached 1377 mAh/g reversible capacity at 0.2C.
These numbers are way above what you get with most lithium-ion batteries, especially when you factor in the cost and sustainability.
Why Should You Care? (For Pros and Curious Kids)
- Engineers & Battery Designers: You can build lighter, longer-lasting batteries for EVs, drones, and grid storage, all while cutting costs.
- Farmers & Sustainability Advocates: Turning waste into high-value products means more revenue and less landfill.
- Investors & Startups: The next big battery breakthrough could come from the cornfields of Iowa or the soybean farms of Illinois.
Step-by-Step Guide: How Biocarbon from Agro-Waste is Used in Li–S Batteries
Step 1: Collect Agro-Waste
- Anything from soybean skins to vegetable sponges can work.
Step 2: Carbonization
- Heat the agro-waste in a low-oxygen environment to turn it into carbon with lots of pores.
Step 3: Activation
- Sometimes, the carbon is treated with chemicals (like phosphoric acid) to make even more pores and add special groups that trap polysulfides.
Step 4: Sulfur Loading
- The biocarbon is mixed with sulfur, which gets trapped in the pores.
Step 5: Assembly
- The sulfur-loaded biocarbon becomes the cathode in a lithium–sulfur battery.
Step 6: Testing and Optimization
- Scientists test the battery’s performance, tweaking the process to get the most energy and longest life.
NTT Docomo Uses Quantum Optimizer to Boost 5G Resource Efficiency by 15%
IonQ To Acquire Oxford Ionics For ~$1.1 B, Expanding Trapped‑Ion Leadership
FAQs About Biocarbon from Agro‑Waste Serves as Sulfur Host in Next‑Gen Li‑S Batteries
Q1: Are lithium–sulfur batteries safe?
A: Yes, they’re generally safer than lithium-ion batteries because they don’t use cobalt and have less risk of overheating.
Q2: When will I see these batteries in my phone or car?
A: They’re still being perfected, but many experts say mass-market products could arrive in the next few years.
Q3: Why not just use regular carbon instead of biocarbon?
A: Biocarbon from agro-waste is cheaper, greener, and often has a better pore structure for trapping sulfur and polysulfides.
Q4: What’s the biggest challenge left?
A: Getting the batteries to last through hundreds or thousands of charge cycles without losing capacity. Managing the “shuttle effect” is key.
Q5: Can farmers really make money from selling agro-waste for batteries?
A: Absolutely! As demand grows, selling waste for battery materials could become a new revenue stream for American farmers.