Precisely Placed Platinum Atoms Boost Catalyst Efficiency and Speed: In recent years, scientists have been pushing the boundaries of catalyst design to create more efficient, durable, and cost-effective materials essential to industries such as energy, automotive, and chemical manufacturing. Among these, platinum-based catalysts are particularly prized for their outstanding performance in accelerating chemical reactions.

Now, a breakthrough from researchers at the University of California, Davis promises to revolutionize the way platinum catalysts work. By precisely placing tiny clusters of platinum atoms on nanoscopic cerium oxide islands, they have achieved remarkable gains in both catalyst efficiency and reaction speed. This cutting-edge approach not only enhances catalytic activity but also increases stability and reduces the amount of platinum needed—addressing one of the biggest challenges in catalyst development: cost.
Precisely Placed Platinum Atoms Boost Catalyst Efficiency and Speed
| Aspect | Details |
|---|---|
| Main Discovery | Atomically confined platinum clusters on cerium oxide nano-islands outperform single atoms and bulk nanoparticles in catalytic activity. |
| Efficiency | Better catalytic performance using fewer platinum atoms—lower cost, higher activity. |
| Stability | Catalyst remains stable under high-temperature, high-pressure industrial conditions. |
| Industrial Use Cases | Hydrogenation, fuel cells, emission controls, petroleum refining. |
| Research Institution | University of California, Davis |
| Published In | Nature Chemistry, 2024 |
| Official Resource | UC Davis Newsroom |
| Keywords | platinum catalyst, catalyst efficiency, hydrogenation catalyst, nanocatalysis, atomically precise platinum |
The precise placement of platinum atoms on cerium oxide nano-islands represents a significant leap in catalyst engineering. By improving atom utilization, stability, and reaction rates, this method offers a more cost-effective and sustainable solution for industries heavily reliant on platinum catalysts. The combination of fundamental chemistry, nanotechnology, and materials science embodied in this discovery could usher in a new era of smarter catalyst design, contributing to greener, cleaner industrial processes worldwide.
Why This Breakthrough Matters: The Role of Platinum Catalysts
Platinum is a noble metal that plays a critical role in various catalytic processes due to its unique chemical properties, including high resistance to corrosion and exceptional ability to facilitate electron transfer. Industries worldwide rely on platinum catalysts for:
- Petroleum refining: speeding up hydrogenation reactions to produce fuels and lubricants.
- Emission control: converting harmful exhaust gases into less toxic compounds in vehicle catalytic converters.
- Fuel cells: enabling efficient hydrogen oxidation and oxygen reduction reactions for clean energy.
- Pharmaceutical synthesis: facilitating key chemical transformations with high selectivity.
Despite its effectiveness, platinum is scarce and expensive, with global prices often exceeding $30,000 per kilogram. Therefore, making platinum catalysts more efficient—so that less metal is needed without sacrificing performance—is a top priority for scientists and engineers.
How Precision Placement Unlocks New Catalyst Performance
Traditional platinum catalysts typically involve bulk nanoparticles or single atoms spread across support materials. While these have their advantages, they also suffer drawbacks:
- Bulk nanoparticles can have many atoms buried inside, not exposed for reaction, reducing atom efficiency.
- Single atoms may be highly active but tend to migrate and form larger clusters under reaction conditions, losing their unique benefits.
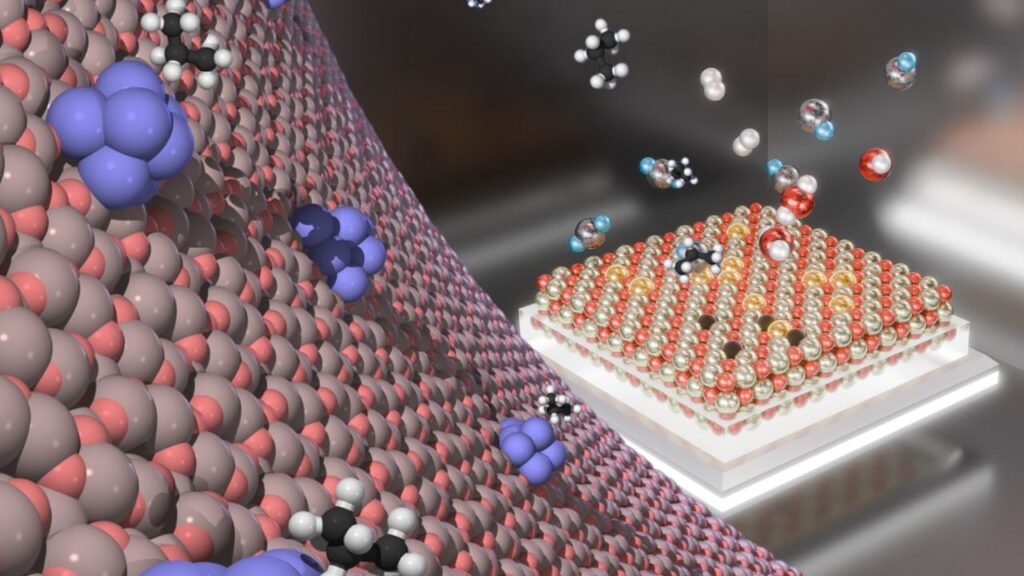
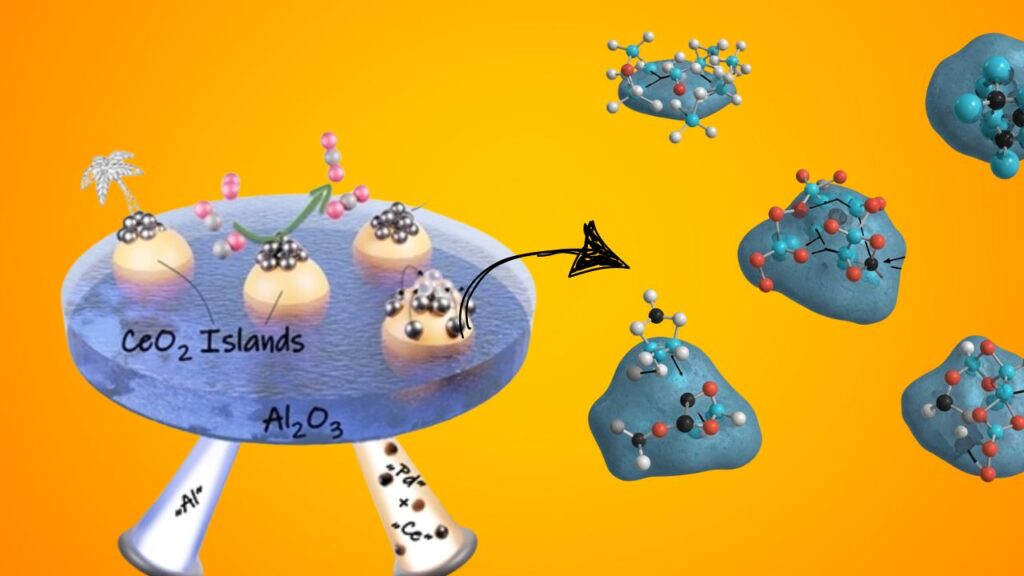
The UC Davis team’s innovation lies in creating atomically precise clusters of 2 to 4 platinum atoms, confined on cerium oxide (CeO₂) nano-islands just a few nanometers wide, which themselves rest on a silica substrate.
Why Cerium Oxide?
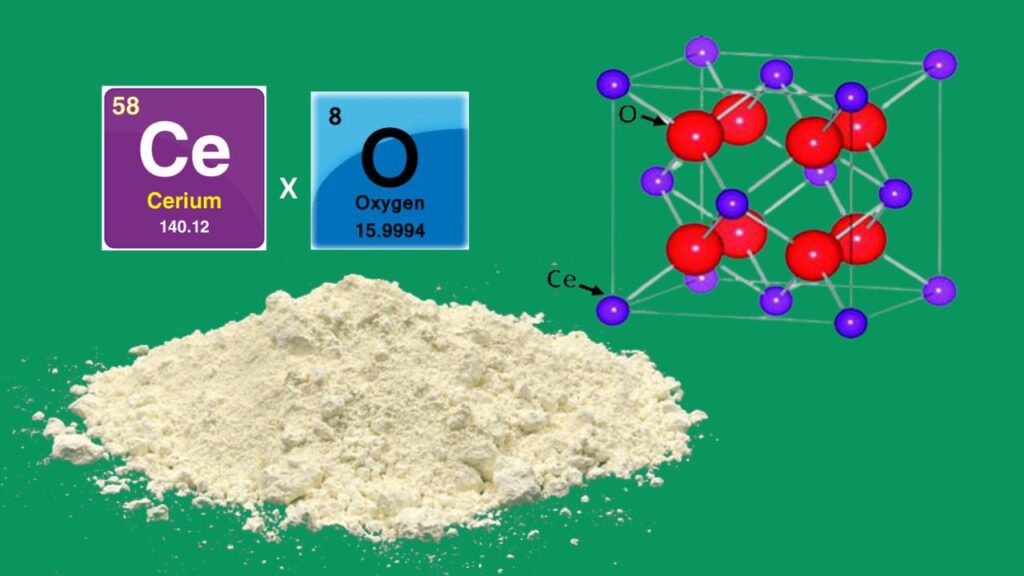
Cerium oxide is a well-known support material in catalysis due to its:
- Oxygen storage capacity: It can easily switch between Ce⁴⁺ and Ce³⁺ oxidation states, providing oxygen for reactions.
- Strong metal-support interaction: Stabilizes small metal clusters and prevents sintering (particle growth).
- Thermal stability: Maintains structure at high reaction temperatures.
By depositing platinum clusters exclusively on these nano-islands, the metal atoms are effectively trapped, preventing aggregation and maintaining their small, highly active size.
Detailed Mechanism of Improved Catalytic Activity
1. Enhanced Surface Atom Utilization
Since the platinum clusters contain only a few atoms, every atom is surface-exposed and able to participate in chemical reactions. This contrasts with larger nanoparticles where a significant portion of atoms are inside the particle and inactive.
2. Synergistic Effects with Cerium Oxide
The cerium oxide support can actively participate by:
- Donating or accepting oxygen species during the catalytic cycle.
- Modulating the electronic properties of platinum atoms to enhance their reactivity.
This synergy improves both the rate and selectivity of hydrogenation reactions.
3. Improved Stability Against Sintering
By anchoring platinum atoms on defined cerium oxide islands, the clusters are less likely to migrate and merge, which commonly happens in traditional catalysts under high temperatures. This results in longer catalyst lifetimes and consistent performance.
Practical Implications and Industrial Relevance
This breakthrough has important real-world implications:
Cost Savings and Resource Efficiency
- Using fewer platinum atoms to achieve higher activity means lower raw material costs.
- Given platinum’s price volatility and scarcity, this could translate to significant savings for industries reliant on platinum catalysts.
Increased Catalyst Durability
- Stability under harsh conditions (high temperatures, reactive gases) means fewer catalyst replacements.
- This reduces downtime and maintenance costs in industrial processes.
Versatility Across Applications
- The technique is relevant not just for hydrogenation but could be extended to other platinum-catalyzed reactions including oxidation, reforming, and fuel cell reactions.
- Cerium oxide is abundant and inexpensive, facilitating scalability.
Supporting Research and Data
The team published their findings in the prestigious journal Nature Chemistry (2024), after rigorous testing involving:
- Turnover frequency (TOF) measurements showing up to a 4-fold increase in reaction rate per platinum atom compared to conventional catalysts.
- Long-term cycling tests confirming no significant loss of activity after hundreds of reaction cycles at temperatures exceeding 300°C.
- Advanced characterization techniques including high-resolution transmission electron microscopy (HR-TEM) and X-ray photoelectron spectroscopy (XPS) to confirm atomic-scale structure and oxidation states.
Step-by-Step Guide to Catalyst Fabrication
For researchers and industry professionals interested in replicating or adapting this catalyst system, the process broadly involves:
- Preparation of Silica Substrate: A high-surface-area silica material is cleaned and pre-treated to support cerium oxide deposition.
- Cerium Oxide Deposition: Controlled growth of nano-islands of cerium oxide is achieved via chemical vapor deposition or wet chemical methods, creating nanometer-sized “patches.”
- Platinum Atom Cluster Deposition: Techniques like atomic layer deposition (ALD) or solution-based methods are used to deposit atomically precise clusters of 2–4 platinum atoms exclusively on these cerium oxide islands.
- Catalyst Activation: Heat treatment under controlled atmosphere stabilizes the catalyst and prepares active sites.
- Catalytic Testing: The catalyst is evaluated under relevant reaction conditions such as ethylene hydrogenation or other hydrogenation processes.
Caltech Achieves Quantum Hyper-Entanglement Using Laser Tweezers: A Landmark in Quantum Science
China Unveils FuXi 2.0 AI-Powered Weather Forecasting Model: A New Era in Meteorology
D-Wave Extends Agreement With Aramco for Quantum Geophysical Optimization
FAQs About Precisely Placed Platinum Atoms Boost Catalyst Efficiency and Speed
What is the main advantage of using clusters instead of single atoms or nanoparticles?
Clusters balance the benefits of single atoms (high atom efficiency) and nanoparticles (stability), while overcoming their individual limitations.
How scalable is this catalyst production method?
The materials (silica, cerium oxide, platinum) and deposition methods used are compatible with current industrial processes, making scale-up feasible.
Can this approach be applied to other precious metals?
Yes, similar confinement strategies may enhance catalysts based on rhodium, palladium, or iridium, expanding the impact across various catalytic applications.
How does this catalyst affect the environment?
Using less platinum reduces mining demand and environmental footprint. Enhanced efficiency leads to lower energy consumption in chemical processes.
Is this technology commercially available?
Currently, it is at an advanced research stage, with pilot projects underway to assess industrial feasibility.