Scientists Explore the Potential of MXenes in Revolutionizing Sustainable Ammonia Production—this headline signals a major shift in how we think about both advanced materials and the future of agriculture and clean energy.
In this in-depth article, we’ll explore what MXenes are, why ammonia production is so crucial for society, how MXenes could transform this process, and what this means for professionals, researchers, and the general public. Whether you’re a curious student, an industry expert, or someone passionate about sustainability, you’ll find clear explanations, practical insights, and authoritative information here.
Table of Contents
Potential of MXenes in Revolutionizing Sustainable Ammonia Production
| Feature/Section | Details & Data |
|---|---|
| What are MXenes? | 2D materials made of transition metal carbides, nitrides, or carbonitrides. |
| Why Ammonia Matters | Essential for fertilizer (feeds 50% of the world’s population), fuels, and chemicals. |
| Current Production Issues | The Haber-Bosch process uses 1-2% of global energy and emits over 500 million tons of CO₂/year. |
| MXene Breakthrough | Can catalyze ammonia production from air and water at room temperature, potentially slashing energy use and emissions. |
| Best Performing MXenes | Ti₃C₂Tₓ and Ti₃CNTₓ show highest efficiency and stability for ammonia synthesis. |
| Professional Impact | Opens new research, green tech, and industrial opportunities in clean energy and sustainable agriculture. |
The exploration of MXenes for sustainable ammonia production marks a transformative moment in green chemistry and materials science. By leveraging the unique properties of these 2D materials, researchers are opening the door to cleaner, more efficient fertilizer production—benefiting farmers, industry, and the environment. While challenges remain, the progress so far is promising, and the potential impact is immense. The future of food and energy could very well be shaped by the tiniest building blocks of matter.
Understanding the Basics: What Are MXenes?
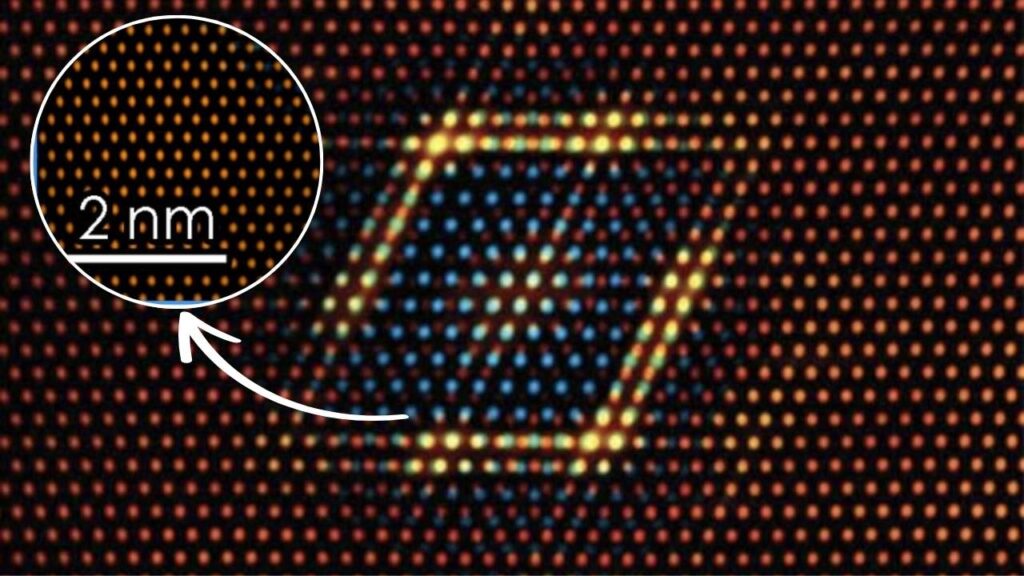
Let’s start with the basics. MXenes are a family of two-dimensional (2D) materials, which means they are made up of layers just a few atoms thick. They’re created by combining transition metals (like titanium or vanadium) with carbon, nitrogen, or both. What makes MXenes extraordinary is their tunable structure: scientists can swap out different atoms or add surface groups to give each MXene unique properties.
Think of MXenes as a set of high-tech building blocks. By changing which “blocks” (atoms) are used, researchers can create materials that conduct electricity, store energy, filter water, or, as we’ll see, catalyze chemical reactions that are vital for life on Earth.
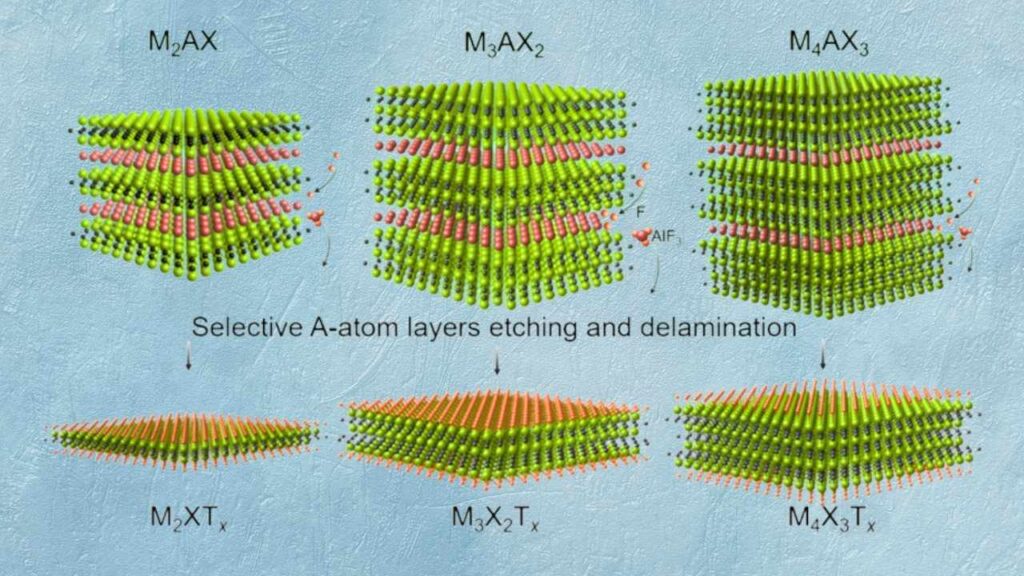
The Discovery and Development of MXenes
Discovered in 2011, MXenes have quickly become one of the most studied materials in nanotechnology. Their layered structure gives them a large surface area and excellent electrical conductivity, making them attractive for a range of applications from batteries to sensors and, most recently, to catalysis.
Why Is Ammonia Production So Important?
Ammonia (NH₃) is much more than a simple gas. It’s a cornerstone of modern civilization, primarily because it’s the main ingredient in fertilizers that help feed about half of the world’s population. Without ammonia-based fertilizers, crop yields would plummet, and global food security would be at risk.
Beyond Fertilizer: Ammonia’s Expanding Role
Ammonia is also used in manufacturing plastics, textiles, explosives, and cleaning products. More recently, it’s being explored as a clean fuel for power plants, ships, and even vehicles, since it can be burned without releasing carbon dioxide if produced sustainably.
The Environmental Cost of Current Ammonia Production
Almost all industrial ammonia is made using the Haber-Bosch process, which combines nitrogen from the air with hydrogen (usually from natural gas) under high temperature and pressure. This process is energy-intensive—using about 1–2% of all global energy—and emits over 500 million tons of carbon dioxide each year. That’s more than the emissions from the entire aviation industry.
MXenes: The Next-Generation Solution
How Do MXenes Help?
MXenes are showing great promise as electrocatalysts—materials that speed up chemical reactions using electricity. Unlike the Haber-Bosch process, which requires high temperatures and pressures, MXene-based systems can potentially produce ammonia at room temperature and atmospheric pressure, using only air, water, and renewable electricity.
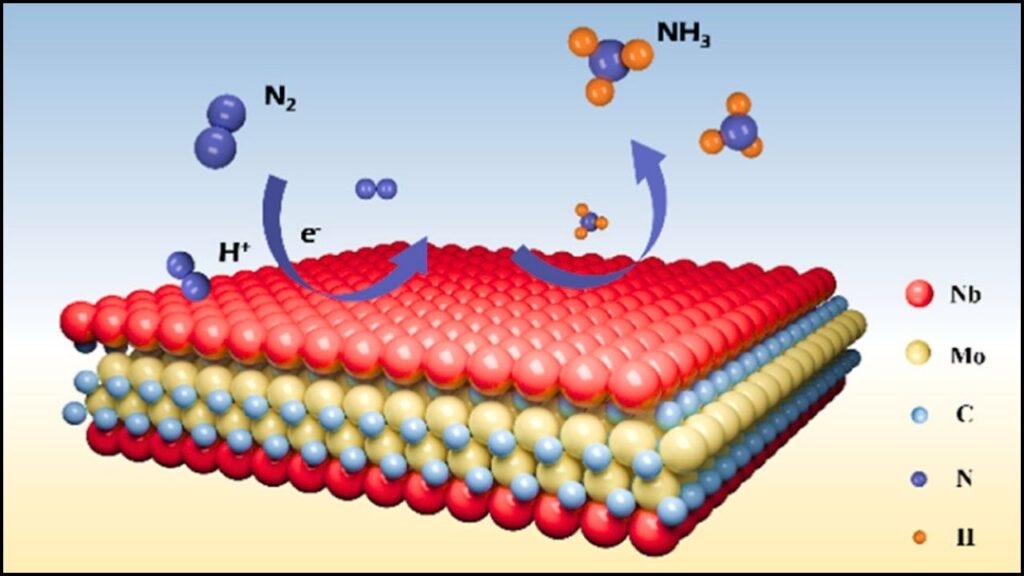
The Basic Process
- Nitrogen and Water Input: Nitrogen is taken from the air, and hydrogen is sourced from water.
- MXene Catalyst: The MXene material helps break the strong bonds in nitrogen molecules, allowing them to combine with hydrogen to form ammonia.
- Powered by Renewable Energy: The process uses electricity, ideally from solar, wind, or hydropower, making it much cleaner than current methods.
What Makes MXenes Special for Ammonia Synthesis?
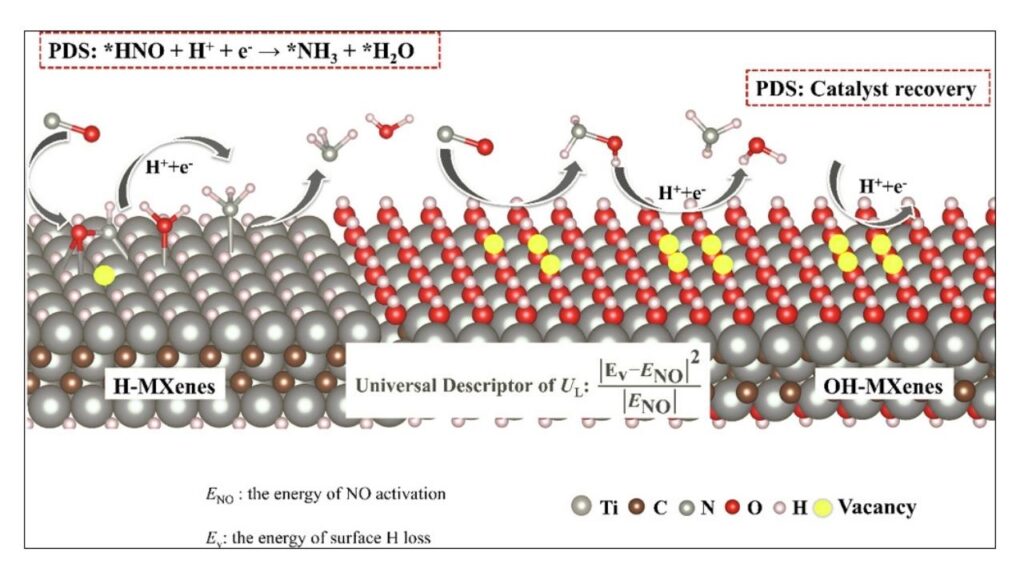
- High Surface Area: Their 2D structure provides more active sites for chemical reactions.
- Excellent Conductivity: MXenes move electrons efficiently, which is crucial for electrocatalysis.
- Tunable Chemistry: Scientists can modify the composition and surface groups of MXenes to optimize their performance for specific reactions.
Recent Breakthroughs
Researchers have found that certain MXenes, such as Ti₃C₂Tₓ and Ti₃CNTₓ, are particularly effective for ammonia synthesis. These materials have shown high efficiency, stability, and selectivity, making them leading candidates for future industrial applications.
The Science Behind the Scenes
Tuning the MXene Structure
One of the most exciting aspects of MXenes is their tunable structure. By changing the type of transition metal or the ratio of carbon to nitrogen, researchers can adjust the material’s electronic and chemical properties. For example, replacing some carbon atoms with nitrogen can change how the MXene interacts with nitrogen molecules, making the catalytic process more efficient.
Surface Termination and Its Impact
MXenes often have various surface groups, such as oxygen, fluorine, or hydroxyl. Studies have shown that oxygen-terminated MXenes are generally more effective at catalyzing ammonia production than those terminated with fluorine. This insight helps guide the design of next-generation catalysts.
Electrochemical Nitrate Reduction
Another promising approach is using MXenes to convert nitrates (NO₃⁻) from water into ammonia. This process not only produces valuable fertilizer but also helps clean up water sources contaminated with excess nitrates—a common problem in agricultural regions.
Stability and Scalability
For any new catalyst to be practical, it must be stable over long periods and scalable for industrial use. Recent experiments have demonstrated that the best-performing MXenes can maintain their activity over many cycles, and efforts are underway to develop cost-effective, large-scale synthesis methods.
Practical Advice: What Does This Mean for Industry and the Environment?
For Farmers and Food Security
- Affordable, Local Fertilizer Production: MXene-based ammonia synthesis could allow fertilizer to be made locally, reducing transportation costs and increasing access for small farmers.
- Sustainable Agriculture: Cleaner production methods mean less environmental impact, supporting long-term food security.
For Energy and Climate
- Significant Emissions Reduction: Replacing the Haber-Bosch process with MXene-based systems could cut global CO₂ emissions by hundreds of millions of tons each year.
- Integration with Renewables: These systems can be powered by intermittent renewable energy sources, making them ideal for a future powered by solar and wind.
For Industry and Professionals
- New Career Opportunities: The rise of MXene technology is creating jobs in materials science, chemical engineering, and green technology sectors.
- Interdisciplinary Collaboration: Success in this field requires teamwork between chemists, engineers, environmental scientists, and policymakers.
Step-by-Step Guide: How MXene-Based Ammonia Production Works
1. Synthesis of MXenes
- Starting Material: Bulk materials known as MAX phases are chemically etched to remove certain layers, leaving behind thin sheets of MXene.
- Surface Modification: The MXene sheets are treated to add or change surface groups, tailoring their properties for catalysis.
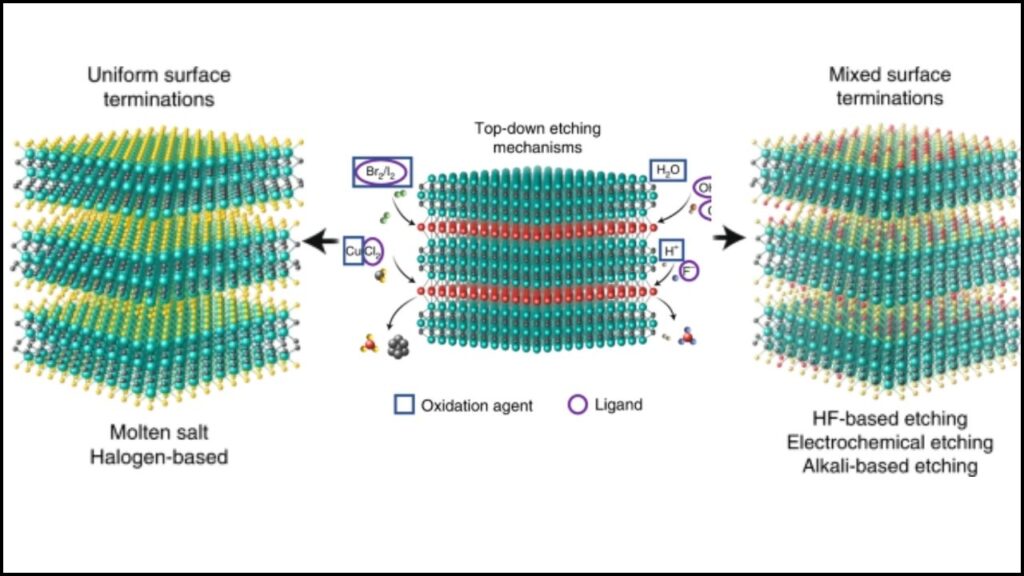
2. Catalyst Testing
- Electrochemical Cell Setup: MXenes are placed in a cell with water and nitrogen (from air or nitrate).
- Application of Electricity: A voltage is applied, and the system is monitored for ammonia production.
3. Performance Evaluation
- Faradaic Efficiency: Measures how much of the electricity is used to make ammonia.
- Yield Rate: The amount of ammonia produced per hour.
- Stability Testing: Ensures the catalyst works over many cycles without degrading.
4. Scaling Up
- Pilot Projects: Promising MXenes are tested in larger reactors.
- Integration with Renewable Energy: Systems are designed to work with solar or wind power for maximum sustainability.
5. Environmental and Economic Assessment
- Life Cycle Analysis: Evaluates the total environmental impact from material production to ammonia synthesis.
- Cost Comparison: Assesses the economic viability compared to traditional methods.
Real-World Example: Ti₃C₂Tₓ and Ti₃CNTₓ
In controlled laboratory experiments, Ti₃C₂Tₓ and Ti₃CNTₓ MXenes have demonstrated high efficiency in converting nitrogen to ammonia using electricity. These materials have maintained their performance over extended periods, showing promise for future industrial adoption. Researchers are now working on scaling up these findings and optimizing the process for commercial use.
Breakthrough Discovery Reveals Surprising New Behaviors in Soft Materials
Scientists Use Lightning to Make Ammonia Out of Thin Air Without Fossil Fuels
CO₂ to Methane Conversion Unlocked by Tweaking Nickel Nanoparticle Shape
FAQs About Potential of MXenes in Revolutionizing Sustainable Ammonia Production
What are MXenes made of?
MXenes are composed of layers of transition metals (such as titanium or vanadium) combined with carbon and/or nitrogen atoms, often with additional surface groups like oxygen or hydroxyl.
Why is ammonia important?
Ammonia is essential for producing fertilizers, which support global food production. It’s also used in many industrial processes and is being explored as a clean fuel.
How does MXene-based ammonia production differ from the Haber-Bosch process?
MXene-based systems can produce ammonia at room temperature and atmospheric pressure using electricity, while the Haber-Bosch process requires high heat, high pressure, and fossil fuels.
Are MXenes commercially available yet?
Most MXene-based ammonia production technologies are still in the research and development phase, but progress is being made toward commercial applications.
What are the environmental benefits?
MXene-based processes could drastically reduce greenhouse gas emissions and energy use compared to traditional ammonia production methods.
Challenges and Future Directions
Technical Hurdles
- Scalability: While laboratory results are promising, producing MXenes on an industrial scale remains a challenge.
- Long-Term Stability: Ensuring that MXene catalysts remain effective over years of use is critical for commercial adoption.
- Selectivity: Improving the selectivity of MXenes for ammonia over unwanted byproducts is an ongoing area of research.
Research and Development
- Material Innovation: Scientists are exploring new MXene compositions and surface modifications to further enhance performance.
- Process Optimization: Engineers are developing new reactor designs and process controls to maximize efficiency and minimize costs.
Policy and Market Considerations
- Regulatory Support: Government incentives and regulations could accelerate the adoption of sustainable ammonia production technologies.
- Market Readiness: Collaboration between academia, industry, and policymakers will be essential to bring MXene-based solutions to market.