Researchers Synthesize Elusive Cyclic Tri-Phosphirenium Ion: The world of chemistry is buzzing with excitement as researchers have finally synthesized the elusive cyclic tri-phosphirenium ion, a breakthrough that promises to reshape our understanding of main-group chemistry. This achievement not only marks a milestone in the synthesis of rare phosphorus compounds but also opens doors to new possibilities in catalysis and molecular design.
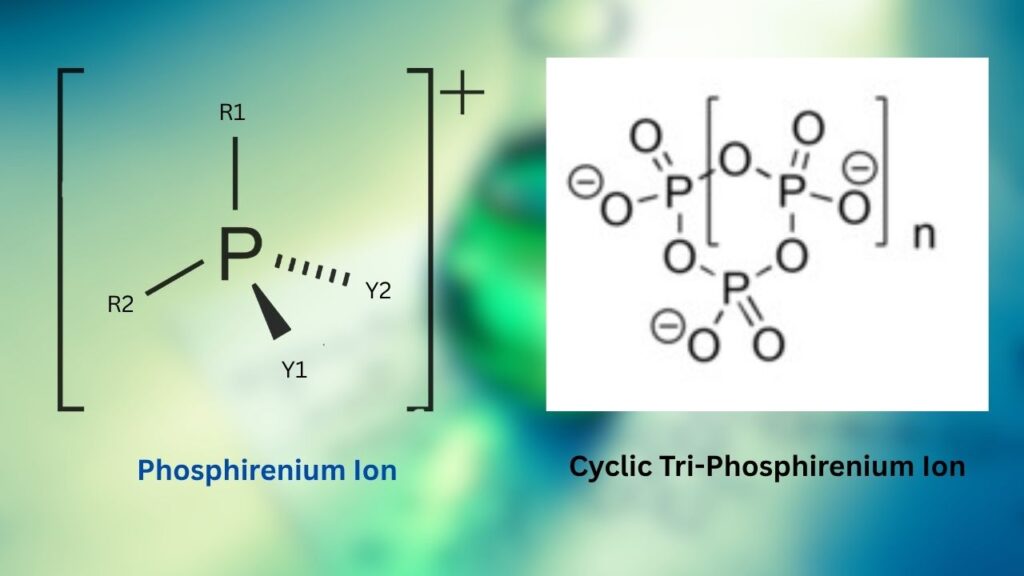
Phosphirenium ions, especially the cyclic tri-phosphirenium type, have long been a “holy grail” for chemists. These tiny, three-membered rings made entirely of phosphorus atoms in a positively charged state are both fascinating and challenging to create. For decades, their fleeting existence was mostly theoretical, with only indirect evidence of their presence in chemical reactions. Now, thanks to innovative synthetic strategies and careful experimentation, these ions have been isolated and studied, bringing the field of main-group chemistry into a new era.
Researchers Synthesize Elusive Cyclic Tri-Phosphirenium Ion
| Feature/Topic | Details & Data |
|---|---|
| What is a tri-phosphirenium ion? | A three-membered ring of phosphorus atoms with a positive charge |
| Why is it important? | Enables new types of catalysis and deepens understanding of main-group chemistry |
| How was it made? | By reacting diphenyl acetylene with secondary phosphine oxide in acetonitrile |
| Key application | Acts as a “masked phosphenium” catalyst for metal-free carbonyl reduction |
| Career relevance | Advances in main-group chemistry, catalysis, and synthetic methodology |
| Official resource | ACS Catalysis Official Website |
The synthesis of the elusive cyclic tri-phosphirenium ion is a landmark achievement in main-group chemistry. It shows that even elements outside the traditional “star players” of chemistry can form complex, useful, and fascinating molecules. As researchers continue to explore its properties and uses, we can expect new breakthroughs in catalysis, materials science, and sustainable chemistry.
What Is a Cyclic Tri-Phosphirenium Ion?
A cyclic tri-phosphirenium ion is a molecule made of three phosphorus atoms connected in a ring, carrying a positive charge. Imagine three friends holding hands in a circle, but instead of people, they’re phosphorus atoms, and the circle is charged like a battery. This structure is extremely rare and unstable, making it very hard to catch and study.
Phosphirenium ions are part of a broader family of phosphorus-based molecules that have unique electronic properties. Chemists have been interested in these ions because they can act as powerful catalysts and intermediates in chemical reactions, especially those that don’t require metals.
Why Is This Discovery Important?
Main-group chemistry focuses on elements like carbon, nitrogen, oxygen, and phosphorus—elements that are everywhere in our lives, from the air we breathe to the DNA in our cells. While transition metals (like iron, copper, and platinum) have traditionally dominated the world of catalysis and advanced materials, main-group elements are catching up.
The synthesis of the cyclic tri-phosphirenium ion is a big deal because:
- It proves that main-group elements can form complex, reactive structures once thought possible only for metals.
- It provides a new “tool” for chemists to design reactions that are more sustainable and less reliant on expensive or toxic metals.
- It helps us understand how phosphorus atoms behave when forced into tiny rings, which can lead to new materials and catalysts.
How Did Researchers Synthesize the Cyclic Tri-Phosphirenium Ion?
The journey to making this ion wasn’t easy. Chemists had to carefully choose the right ingredients, conditions, and techniques. Here’s a simplified step-by-step guide to how they did it:
1. Choosing the Starting Materials
Researchers started with secondary phosphine oxide (a molecule containing phosphorus, hydrogen, and oxygen) and diphenyl acetylene (a molecule with two benzene rings and a triple bond).
2. Selecting the Right Activator
They needed a way to “activate” the phosphorus compound so it would react with the acetylene. After trying several options, they found that triflic anhydride (Tf₂O) worked best, but only under precise conditions.
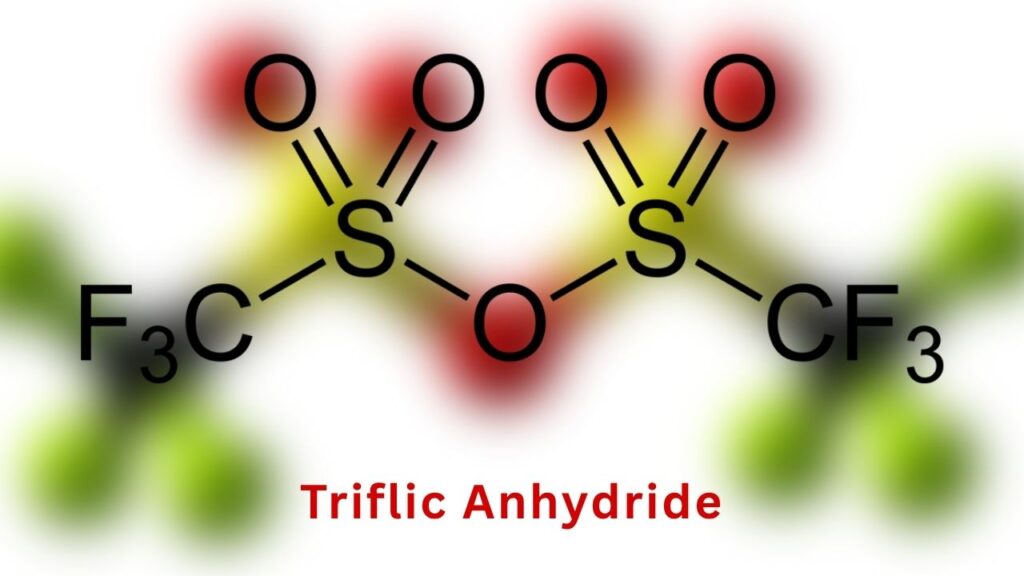
3. Optimizing the Reaction Conditions
- The best results came when the reaction was run in acetonitrile (CH₃CN) at 60°C for 30 minutes.
- Under these conditions, the tri-phosphirenium ion formed efficiently, as confirmed by NMR spectroscopy (a technique that lets chemists “see” the atoms in a molecule).
4. Characterizing the Product
Once formed, the ion was isolated and studied using advanced techniques like X-ray crystallography (which shows the exact arrangement of atoms) and NMR. The data matched what chemists expected for a cyclic tri-phosphirenium ion.
Practical Applications: Why Should We Care?
Catalysis Without Metals
One of the most exciting uses for this new ion is as a catalyst—a substance that speeds up chemical reactions without being consumed. Traditionally, many catalysts are made from rare or toxic metals. The tri-phosphirenium ion, however, acts as a “masked phosphenium” source, meaning it can release a highly reactive phosphorus species that helps transform other molecules.
Example:
In the reduction of carbonyl compounds (like turning a ketone into an alcohol), the tri-phosphirenium ion can help transfer hydride ions (from silanes) to the carbonyl group. This process is important in making pharmaceuticals and fine chemicals, and doing it without metals is a big win for green chemistry.
A Springboard for New Chemistry
Because the tri-phosphirenium ion is so reactive, it can also be used to make other unusual phosphorus compounds. This opens up new pathways for creating molecules with unique properties—potentially useful in electronics, materials science, and medicine.
Breaking Down the Science: Easy-to-Follow Guide
What Makes the Tri-Phosphirenium Ion Special?
- Small Ring, Big Strain: The three-membered ring is under a lot of “strain,” like a tightly wound spring. This makes it eager to react with other molecules.
- Positive Charge: The ion’s positive charge makes it a strong electrophile, meaning it loves to attract electrons from other molecules.
- Masked Reactivity: It can “hide” its true reactivity until the right moment, acting as a safe storage form for highly reactive phosphorus.
How Do Chemists Use It?
- As a Pre-Catalyst: The ion can be added to a reaction mixture, where it releases the active catalyst when needed.
- For Ring-Opening Reactions: Its strained ring can be “opened” by other molecules, leading to new products and transformations.
- For Making New Materials: The unique bonding in the ion can inspire the design of new polymers or electronic materials.
Biocarbon from Agro‑Waste Serves as Sulfur Host in Next‑Gen Li‑S Batteries
FAQs About Researchers Synthesize Elusive Cyclic Tri-Phosphirenium Ion
Q1: What is a phosphirenium ion?
A: It’s a three-membered ring made of phosphorus atoms, carrying a positive charge. Think of it as a tiny, charged triangle of phosphorus.
Q2: Why is the cyclic tri-phosphirenium ion so hard to make?
A: The ring is very strained and unstable, so it usually breaks apart before chemists can study it. Careful choice of reactants and conditions is needed to catch it.
Q3: What are the main uses of this ion?
A: It acts as a catalyst for important reactions, especially those that don’t need metals, and helps make new types of phosphorus compounds.
Q4: How do scientists know they made the right molecule?
A: They use advanced tools like NMR spectroscopy and X-ray crystallography to “see” the atoms and confirm the structure.