Gold—the lustrous, yellow metal prized in jewelry, electronics, and even Olympic medals—has just rewritten the rules of physics. In July 2025, an international team of scientists heated gold to an astonishing 19,000 Kelvin—14 times hotter than its normal melting point—and, for a fleeting instant, it didn’t melt. This mind-boggling discovery, published in Nature, overturns decades-old theories about the limits of solids and opens new frontiers in science and technology. Let’s unpack what happened, why it matters, and what it means for our understanding of the world.

Scientists Superheated Gold to 14 Times Its Melting Point
| Category | Details |
|---|---|
| Gold’s normal melting point | 1,337 Kelvin (1,064°C, 1,947°F) |
| Superheated temperature achieved | 19,000 Kelvin (18,726°C, 33,740°F) |
| Duration at superheated state | 45 femtoseconds (0.000000000000045 seconds) |
| Research team | Led by the University of Nevada, Reno, with SLAC National Accelerator Laboratory, University of Oxford, Queen’s University Belfast, and others |
| Method | Ultrathin gold foil, flash-heated by a high-power laser, probed by ultrabright X-rays for direct temperature and structure measurement |
| Outcome | Gold remained a solid crystal at 14 times its melting point, contrary to the long-standing “entropy catastrophe” theory |
| Official source | Superheating gold beyond the predicted entropy catastrophe threshold, Nature, 23 July 2025 (https://www.nature.com/articles/s41586-025-09253-y) |
Gold’s record-breaking feat—remaining solid at 14 times its melting point—is a triumph of curiosity, teamwork, and technological innovation. It shows that the universe is full of surprises, and that science is never “finished.” Whether you’re a kid exploring at home, a student inspired by discovery, or a professional pushing the boundaries of your field, this story is a reminder: the impossible is just waiting for someone to try.
The Story Behind the Science: How Did They Do It?
Gold’s Normal Behavior
First, let’s set the stage. In everyday life, when you heat gold, it expands a bit, then—once it hits 1,337 Kelvin—it melts and turns into a shiny liquid. That’s what happens in jewelry workshops or even when gold is recycled in factories. But this experiment was anything but everyday. The scientists wanted to break the rules—and they did.
The Experiment Step by Step
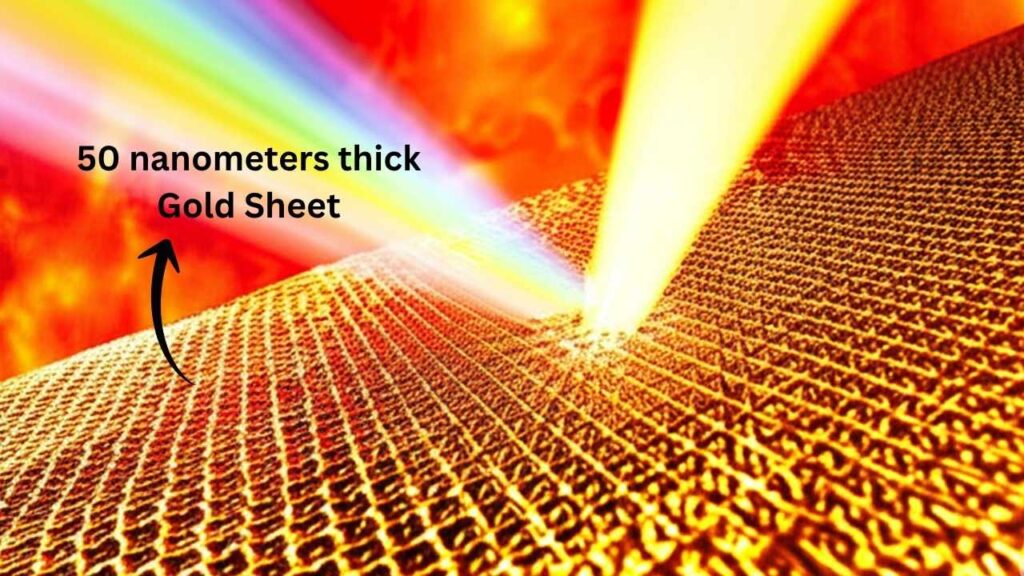
Step 1: Making Gold Paper-Thin
The team started with a gold sample just 50 nanometers thick—thinner than a human hair by a thousand times. Why so thin? When you make something this tiny, heat zips through it almost instantly, giving every atom a fast, uniform blast.
Step 2: The World’s Fastest Flash-Heater
Next, they fired one of the planet’s most powerful lasers at the gold. The laser pulse lasted just 45 femtoseconds—that’s less than a trillionth of a second. In that blink, the laser dumped a colossal amount of energy into the gold, heating it beyond anything ever achieved in a solid.
Step 3: “Snap!”—The Fastest X-ray Camera
Immediately after the laser struck, the team hit the gold with ultrabright X-rays. These acted like a “camera” moving at the speed of light, capturing what was happening inside the gold—atom by atom. The X-rays showed the atoms’ positions and how fast they were vibrating (i.e., how hot they were).
Step 4: Measuring the “Impossible”
The gold atoms were moving so furiously that the calculated temperature was 19,000 Kelvin—hotter than most stars in the universe. But the X-rays confirmed: the gold was still a solid crystal, not a liquid, plasma, or gas. This was totally unexpected.
Step 5: The Split-Second Before Chaos
Almost instantly, the gold did melt and literally exploded—but for that brief 45 femtoseconds, scientists had a window into a world where the old rules of matter didn’t apply.
Why Is This So Shocking? The “Entropy Catastrophe” Explained
The “Entropy Catastrophe” Theory
For over 40 years, textbooks taught that solids can only exist below a certain temperature limit. This limit, called the “entropy catastrophe”, was thought to be about three times the melting point of a material (for gold, ~4,000 Kelvin). Above this, the solid’s disorder (entropy) equals that of the liquid, so the solid must melt—no matter what.
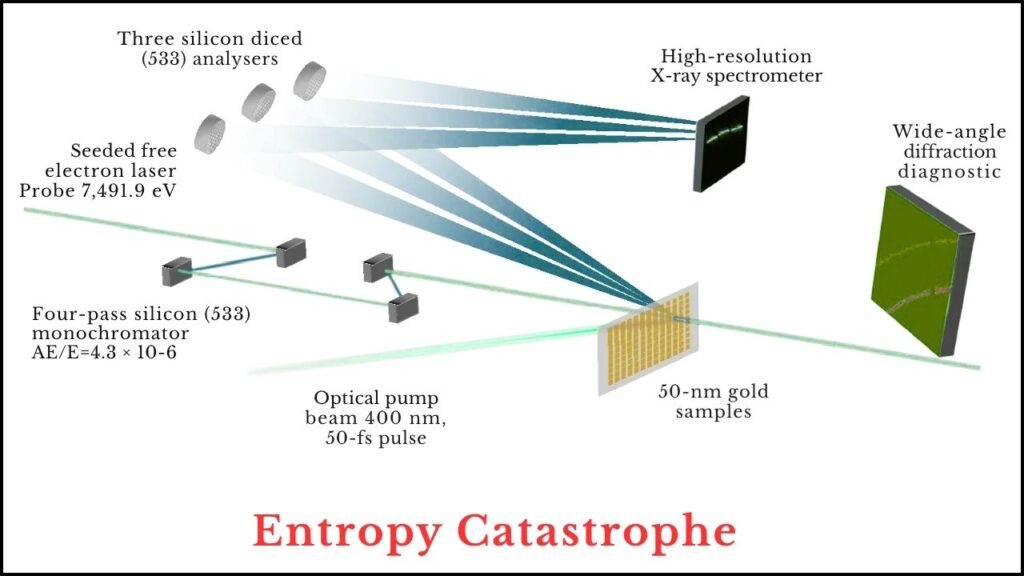
This experiment blew past that limit by a factor of almost five. Gold remained solid at fourteen times its melting point.
How Did Gold “Beat” the Catastrophe?
The answer was speed. The ultrafast laser heated the gold so quickly that the atoms didn’t have time to fall into chaotic, liquid-like motion. The gold was “frozen” in its solid state—not because of cold, but because of the blinding speed of heating. This is a bit like freezing a drop of water by heating it so fast it can’t evaporate before you “freeze” it in place.
What Does This Mean for Science? Rethinking the Rules
A New View of the Limits of Solids
This discovery forces us to rethink what a “solid” can be. We now know that how fast you heat something is just as important as how much you heat it. The entropy catastrophe limit still holds for “slow” heating, but for ultrafast processes—lasting just femtoseconds—solids can survive at temperatures once thought impossible.
Implications for Astronomy, Fusion, and More
This isn’t just academic. Inside stars, planets, and fusion reactors, matter is often both super hot and super dense. This state, called “warm dense matter”, is hard to study on Earth—until now.
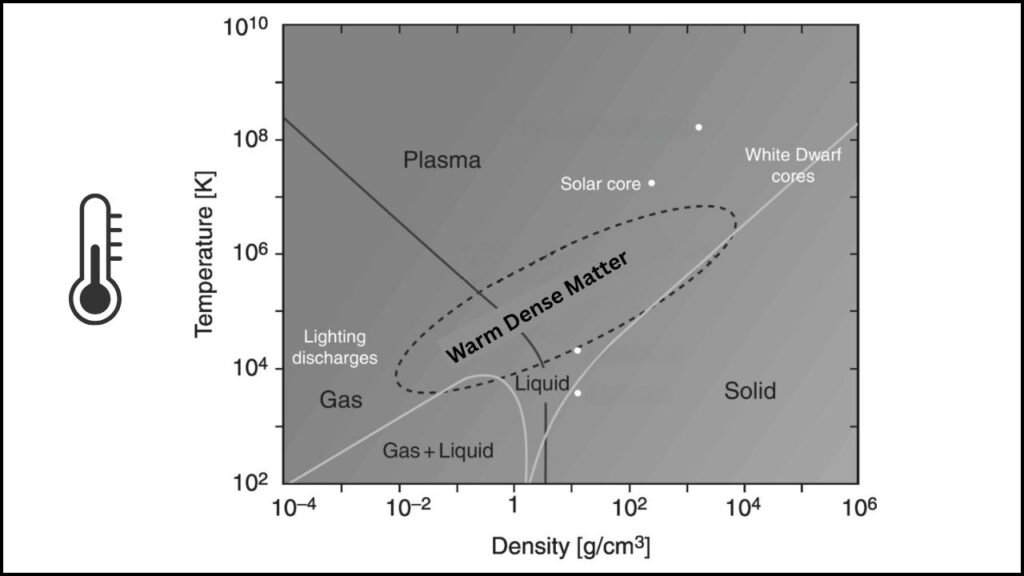
The gold experiment gives us a way to create and measure these extreme conditions in the lab, helping us understand what happens in the hearts of suns and inside Jupiter.
Fusion reactors, which promise clean, limitless energy, operate at these extreme temperatures. If we can control how solids behave when heated ultrafast, we might find new ways to protect reactor walls, improve efficiency, or discover new materials for space travel.
How Was This Experiment Conducted? A Detailed Guide
1. Preparing the Gold
The scientists started with high-purity gold, thinning it down to a film just 50 nanometers thick. This is so thin that heat travels through it almost instantly, ensuring that the whole sample is heated evenly—no “hot spots” or cold patches.
2. The Laser Blast
A high-energy laser pulse, lasting only 45 femtoseconds, was aimed at the gold. This laser delivers an incredible amount of energy in an almost unimaginably short time. The gold doesn’t have time to expand, melt, or even realize it’s being heated—it just gets hot, fast.
3. X-ray Probing
Right after the laser, the gold was hit with ultrabright X-rays from a synchrotron at SLAC National Accelerator Laboratory. These X-rays provided a real-time “snapshot” of the gold’s atomic structure, allowing the team to measure both the position and motion of the atoms.
4. Measuring Temperature and State
From the X-ray data, scientists could calculate the temperature (19,000 K) and confirm that, despite the heat, the gold atoms were still arranged in a crystalline lattice—signature of a solid. They also saw that, as expected, the gold soon melted and exploded—but for that fleeting instant, it was a superheated solid.
5. Double-Checking and Confirming
The team repeated the experiment, checked their calculations, and even invited other labs to confirm their results. This level of rigor is standard in major scientific discoveries, ensuring that the findings are reliable and repeatable.
A Personal Touch: Meet the Scientists Behind the Discovery
Dr. Tom White from the University of Nevada, Reno, led the team. He described the moment they first saw the data as a mix of excitement and disbelief. “We thought there must be a mistake—this wasn’t supposed to be possible.” At SLAC, where the X-ray probing took place, veteran physicists joked about how “unthinkably hot” their experiment was.
This story is a reminder that science is alive with curiosity and doubt. The best discoveries happen when researchers are willing to question textbooks and try something new—even if, at first, it seems impossible.
Practical Takeaways for Everyone
For Kids and Families:
You can experiment with the idea of fast vs. slow heating at home. Try melting chocolate in the microwave (fast) versus in a double boiler (slow). Notice how the speed of heating changes what happens! This is a simple version of what the scientists did—just with gold and a much bigger “microwave.”
For Students and Teachers:
This experiment is a perfect example of how science is always changing. Old theories get updated with new data, and breakthroughs happen when creative minds ask, “What if?” If you’re interested in joining scientific research, look into fields like laser physics, materials science, and ultrafast spectroscopy.
For Professionals:
If you work in energy, aerospace, or technology, this research shows that ultrafast heating can reveal new states of matter and push the limits of what materials can do. Collaborating with national labs (like SLAC) and universities can accelerate your own research and help you find practical applications for these discoveries.
FAQs About Scientists Superheated Gold to 14 Times Its Melting Point
Q: Did scientists really break the laws of physics?
A: No, the fundamental laws of physics remain intact. Instead, scientists discovered that how quickly you heat something matters as much as how much you heat it. The “entropy catastrophe” is still valid for slow heating, but ultrafast processes can push solids into new, unexplored territory.
Q: How long did the gold stay solid at this temperature?
A: Only 45 femtoseconds—less than a trillionth of a second. But with today’s technology, that’s plenty of time to take precise measurements and confirm what’s happening.
Q: Could we see superheated metals in everyday life?
A: Not directly—these conditions exist only in extreme environments or in advanced laboratories. However, this research helps us understand stars, planets, and fusion reactors, which could lead to new technologies in the future.
Q: What’s next for this kind of research?
A: Scientists will try this with other metals and materials to see if the effect is universal. They’ll also use ultrafast heating to study warm dense matter, which is crucial for developing fusion energy and understanding astrophysical phenomena.
Q: Is this discovery useful for making new jewelry?
A: Probably not for jewelry, which is made from large, solid pieces at much lower temperatures. But the science could inspire new materials for electronics, aerospace, or energy applications.
Q: Could this lead to new scientific theories or textbooks?
A: Absolutely. Major discoveries like this often lead to revised theories and updated textbooks, especially in the fields of solid-state physics and condensed matter.
The Bigger Picture: Why This Matters
Challenging Old Assumptions
This experiment didn’t just break a temperature record—it broke a mental barrier. For decades, researchers thought they knew the ultimate limits for how hot a solid could be. Now, those limits are in doubt, and the door is open to even wilder experiments with other materials and under even more extreme conditions.
New Tools for Science
Ultrafast lasers and X-ray facilities are now essential tools for investigating the frontiers of matter. They allow us to create conditions that were once only possible in stars or at the centers of planets—right here on Earth. This is a new era for experimental physics.
Applications Beyond the Lab
From fusion energy to planetary science, the implications are vast. Understanding how materials behave at extreme heat and pressure—especially for brief instants—could be key to developing new energy sources, protecting spacecraft, or even simulating the birth of stars.