Simple Chemical Solution Produces Ultra-Thin Materials: Imagine a world where your phone is thinner, your computer is faster, and solar panels are so light they can be rolled up like paper. This isn’t science fiction—it’s the promise of ultra-thin materials created using simple chemical solutions. These materials are revolutionizing electronics, energy, and technology as we know it.
In this article, we’ll explore how scientists use straightforward chemical processes to make these incredible materials, why they matter, and how they’re changing the future. Whether you’re a curious student, a tech enthusiast, or a professional in the field, you’ll find practical advice, clear examples, and expert insights on this cutting-edge topic.
Simple Chemical Solution Produces Ultra-Thin Materials
| Feature or Fact | Details & Data | Career/Professional Info |
|---|---|---|
| What are Ultra-Thin Materials? | Materials just a few atoms thick, often less than 2 nanometers (nm) | Used in electronics, batteries, sensors, and more |
| How are they made? | Chemical solution synthesis, soft-chemical routes, liquid–liquid interface | Scalable, cost-effective, eco-friendly methods |
| Key Example: 2D Triazine Polymers | One-step, solvent-free reaction; 85% yield; thickness <2 nm; high flexibility | Used in advanced batteries, flexible electronics |
| Electronic Properties | High conductivity, tunable bandgaps, quantum effects | Enables faster, more efficient devices |
| Industrial Scale Production | Methods allow large-scale, uniform, high-quality nanocrystals | Critical for manufacturing and commercial applications |
| Career Impact | Skills in nanomaterials synthesis, materials science, and chemical engineering are in high demand | Opportunities in electronics, energy, research, and manufacturing |
The ability to produce ultra-thin materials with powerful electronic properties using simple chemical solutions is transforming technology. From flexible electronics to advanced batteries, these materials are unlocking new possibilities for innovation and efficiency. As research continues, the impact of ultra-thin materials will only grow—reshaping industries and everyday life.
What Are Ultra-Thin Materials?
Ultra-thin materials are substances that are only a few atoms or molecules thick. To put this in perspective, a single sheet of regular paper is about 100,000 nanometers thick. Ultra-thin materials can be less than 2 nanometers thick—so thin you’d need a super-powered microscope to see them!
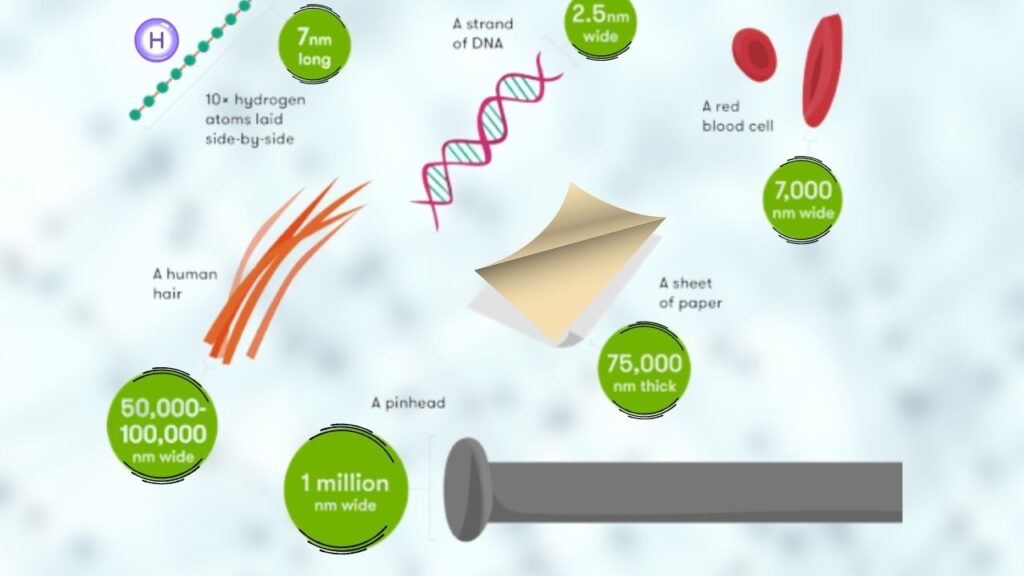
These materials can be made from metals, semiconductors, or polymers. Because they’re so thin, they often have unique properties that you don’t find in bulk materials. For example, they might conduct electricity better, bend without breaking, or react differently to light.
How Are Ultra-Thin Materials Made Using Simple Chemical Solutions?
The secret to making these materials is chemical solution synthesis—a process where scientists mix chemicals in a liquid to form ultra-thin films or nanostructures.
Let’s break down how this works:
1. Choosing the Right Ingredients
Scientists start with carefully selected chemicals that will react to form the desired material. For example, to make copper sulfide (CuS) nanosheets, they use copper chloride and sulfur.
2. Mixing in Solution
The chemicals are mixed in a liquid, like water or an organic solvent. Sometimes, the mixture is heated or stirred to help the reaction along. The liquid environment allows atoms to move freely and form thin layers or wires.
3. Controlling the Reaction
By adjusting things like temperature, concentration, and pH, scientists can control the thickness, size, and shape of the resulting material. For example, using a soft-template strategy, researchers can make ultrathin metal sulfide nanocrystals with thicknesses as low as 3.2 nm.
4. Harvesting the Ultra-Thin Material
Once the reaction is done, the ultra-thin material is separated from the solution, often by filtering or letting it settle. Sometimes, it’s washed and dried to remove any leftover chemicals.
5. Processing for Use
The ultra-thin materials can be made into films, wires, or other shapes depending on their intended use. For example, they might be layered onto flexible plastic to make bendable electronics.
Why Are Ultra-Thin Materials So Special?
Ultra-thin materials have powerful electronic properties that set them apart:
- High Electrical Conductivity: Electrons can move more freely, making them ideal for fast, efficient circuits.
- Tunable Bandgaps: Scientists can adjust how these materials interact with electricity and light, which is crucial for solar cells and sensors.
- Quantum Effects: At such thin scales, materials can show quantum behaviors, leading to new possibilities in computing and information storage.
- Flexibility and Strength: Despite being incredibly thin, many of these materials are flexible and strong, perfect for wearable or foldable devices.

Example:
A recent breakthrough involved making 2D triazine-based polymers using a one-step, solvent-free chemical reaction. The resulting nanosheets were less than 2 nm thick, highly crystalline, and could be used in flexible batteries with outstanding performance—delivering a specific capacity of 356 mAh/g and maintaining 95.1% capacity after 1,000 cycles.
Real-World Applications: Where Are Ultra-Thin Materials Used?
Ultra-thin materials are already making waves in several industries:
Electronics
- Flexible Screens: Imagine phones and tablets that can bend without breaking.
- Faster Chips: Ultra-thin materials can make computer chips smaller, faster, and more energy-efficient.
Energy
- Advanced Batteries: 2D polymers are being used to create batteries that last longer and charge faster.
- Solar Cells: Ultra-thin films can capture more sunlight, making solar panels lighter and more efficient.
Sensors and Medical Devices
- Wearable Health Monitors: Thin, flexible sensors can stick to your skin and track your health in real time.
- Smart Bandages: Ultra-thin films can deliver medicine directly to wounds or monitor healing.
Environmental Solutions
- Water Purification: Thin films can filter out pollutants at the molecular level.
- Efficient Catalysts: Ultra-thin materials can speed up chemical reactions for cleaner energy production.
Step-by-Step Guide: Making Ultra-Thin Materials with Chemical Solutions
Here’s a simplified guide to how professionals create these materials:
Step 1: Select Precursors
Choose chemicals that will react to form the desired ultra-thin material. For example, to make silver nanowires, use silver nitrate and a reducing agent.
Step 2: Prepare the Solution
Dissolve the chemicals in a suitable solvent. The choice of solvent affects the reaction and the final material’s properties.
Step 3: Control the Environment
Adjust temperature, pH, and concentration. For example, a three-step, seed-mediated process can produce ultrathin silver nanowires with diameters as small as 17 nm.
Step 4: Initiate the Reaction
Start the chemical reaction by mixing the solutions or heating them. Sometimes, catalysts like AlCl₃ are used to speed up the process and control the structure.
Step 5: Isolate the Product
Separate the ultra-thin material from the solution, often by filtration or precipitation.
Step 6: Purify and Process
Wash and dry the material. It can then be processed into films, wires, or other useful forms.
Practical Advice for Professionals and Students
- Stay Curious: The field of ultra-thin materials is evolving rapidly. Keep up with the latest research and breakthroughs.
- Learn the Basics: Understanding chemistry and materials science is key. Courses in nanotechnology and chemical engineering are valuable.
- Experiment Safely: Always follow safety protocols when handling chemicals.
- Collaborate: Work with experts in physics, engineering, and computer science to unlock new applications.
- Think Big (and Small!): From solar panels to medical devices, ultra-thin materials have limitless potential.
Microsoft Debuts Majorana 1 Processor: Topological Qubits Pave Way for Million-Qubit Quantum Chips
Scientists Discover Why Symmetrical Crystals Absorb Light Unevenly
Fluorescent Molecules Withstand Extreme Environments Using New Technique
FAQs About Simple Chemical Solution Produces Ultra-Thin Materials
Q1: What makes ultra-thin materials different from regular materials?
Ultra-thin materials are just a few atoms thick, giving them unique properties like higher conductivity, flexibility, and special quantum effects not found in thicker materials.
Q2: Are these materials safe to use?
When handled properly, ultra-thin materials are safe. However, like all chemicals and nanomaterials, they require careful handling and disposal.
Q3: Can I make ultra-thin materials at home?
While the basic chemistry is simple, making high-quality ultra-thin materials requires precise control and specialized equipment found in professional labs.
Q4: What careers use ultra-thin materials?
Careers in electronics, energy, research, and manufacturing increasingly rely on experts in ultra-thin materials and nanotechnology.